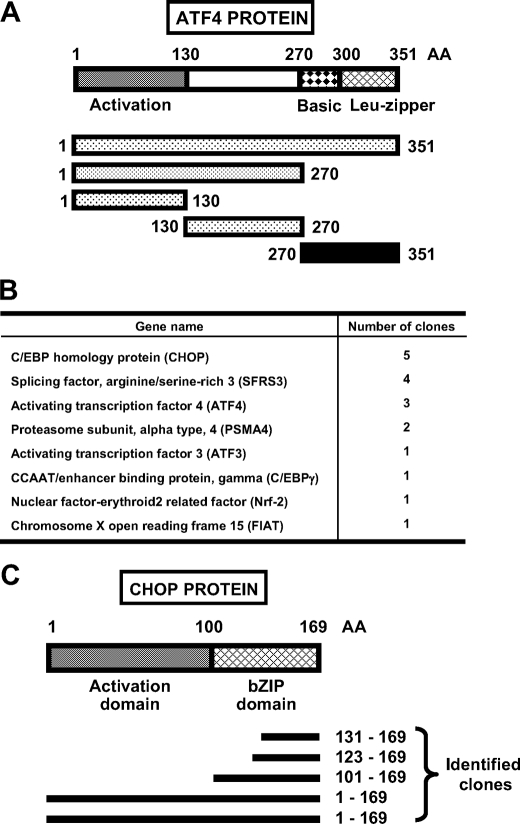
FIGURE 1.
Identification of CHOP as an ATF4 binding partner by yeast two-hybrid screening. A, diagram shows the entire domain structure of the 351 amino acids (AA) that make up the ATF4 protein and the fragments used as bait for yeast two-hybrid screening. For the fragments, the stippled sections indicate the ATF4 regions that exhibited self-activation when tested. The black bar (aa 270-351) in A, corresponding to the basic leucine-zipper domain, was the sequence used to obtain the positive clones shown in B. C, diagram shows the domain structure of CHOP protein, and the black bars below indicate the amino acid sequence of different CHOP clones identified in the yeast two-hybrid screening. The indicated N-terminal (aa 1-100) and C-terminal (aa 100-169) are the CHOP fragments overexpressed to obtain the data in Fig. 7.