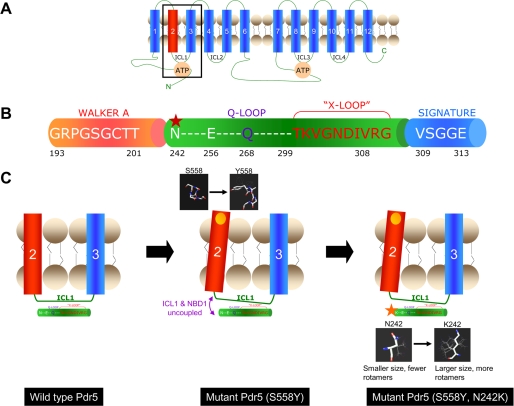
FIGURE 10.
Model for the interaction of Ser-558 and N242K and the signaling interface for coupling ATP hydrolysis to the transport process. A, two-dimensional topological model of Pdr5 showing the 12 transmembrane helices, the two NBDs, and the four intracellular loops (ICL 1–4). The boxed area includes transmembrane helices 2 and 3 and the ICL1 which connects them. The interactions of these domains with the NBD are shown in greater detail in C. B, illustration depicting part of the N-terminal NBD of Pdr5 from the Walker A region to the signature sequence. The Q-loop and “x-loop,” which have been implicated in the mechanical transmission of conformational changes between the NBDs and TMDs, lie between the Walker A and Signature sequence of ABC transporters. The location of N242K, identified in a mutant screen in this study, is shown by a red star. Note that this residue as well as Glu-256 which co-varies with Ser-558 in correlated mutation analysis (see text) are located slightly upstream of the Q-loop. C, left panel shows the TMH2 and -3 of Pdr5 linked by the ICL1 (the boxed area in A). The x-ray crystallographic structure of the ABC transporter Sav1866 describes the “x-loop” in the NBD, which is implicated in the coupling of NBDs and TMDs via the ICL1 (10). The x-loop lies between the Walker A and signature regions of the NBD; the Q-loop also lies in this region and has been implicated in communication between the NBDs and transport sites (34). The central panel shows that the S558Y mutation (yellow circle) involves replacement with a bulkier residue that could result in the tilting of TM helix 2 (see structures in the black boxes for comparison). As the TM helix 2 is directly linked to the ICL1, the interface between the ICL1 and the NBD1 could be disrupted (purple arrows). This is consistent with our results that demonstrate that the S558Y has no effect on the affinity of Pdr5 for nucleotide or transport substrate but shows complete loss of transport activity because the NBD and transport-substrate sites are uncoupled. The right panel shows the S558Y,N242K double mutant. The structures show that the lysine that replaces the asparagine has more rotamers and a potentially longer reach. It is plausible that this change reestablishes the contacts between NBD1 and ICL1 (red arrow).