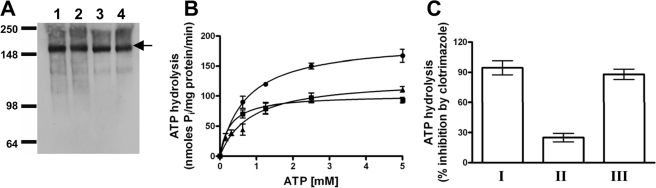
FIGURE 8.
ATP hydrolysis mediated by the S558Y,N242K double mutant of Pdr5. A, immunoblotting of purified plasma membrane vesicles of WT and mutant Pdr5s was carried out as described in Fig. 3A. Vesicles were prepared from yeast strains bearing double copies of WT and mutant Pdr5s. Lane 1, WT Pdr5; lane 2, mutant Pdr5, S558Y; lane 3, mutant Pdr5, N242K; lane 4, mutant Pdr5, S558Y,N242K (clo-3-0). B, Michaelis-Menten plot of Vi-sensitive ATPase activity in purified PM vesicles of clo-3-0 (▴) compared with WT (•) and S558Y (▪). The mean values from three independent experiments are shown, and the error bars represent the S.D. C, Vi-sensitive ATP hydrolysis was measured as described under “Experimental Procedures” in the absence or presence of 25 μm clotrimazole. The mean values for the percent inhibition of ATPase activity by clotrimazole in WT Pdr5 (bar I), mutant Pdr5, S558Y (bar II), and S558Y,N242K (bar III) are shown. Error bars represent the S.D.