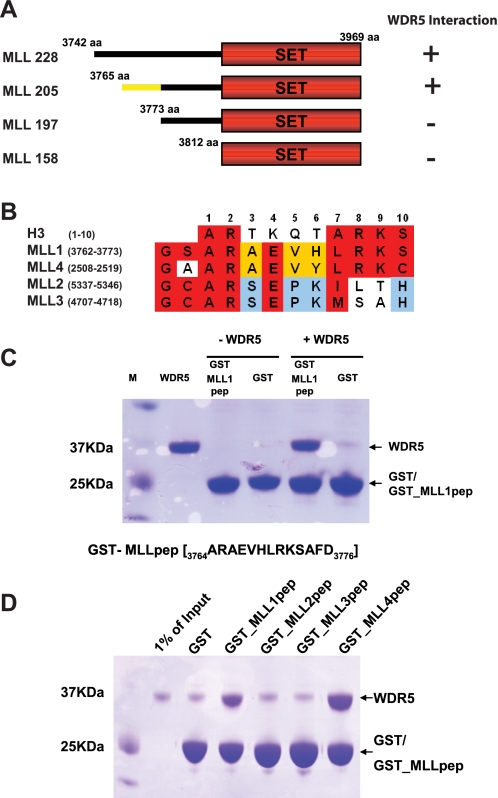
FIGURE 1.
MLL1 interacts with WDR5 through a histone H3-like motif. A, a series of deletion mutants of MLL1 was generated and tested for binding ability to WDR5 by gel filtration chromatography. MLL205 is the minimal construct that interacts with WDR5. B, the sequence in MLL1 required for WDR5 interaction contains a histone H3-like motif and is modestly conserved among MLLs. C, the histone H3-like motif in MLL1 was tested for binding to WDR5. The histone H3-like motif in MLL1 is sufficient to interact with WDR5. D, the histone H3-like motifs in MLL1, MLL2, MLL3, and MLL4 were tested for binding to WDR5 with GST pull-down experiments. Only MLL1 and MLL4 are able to bind WDR5.