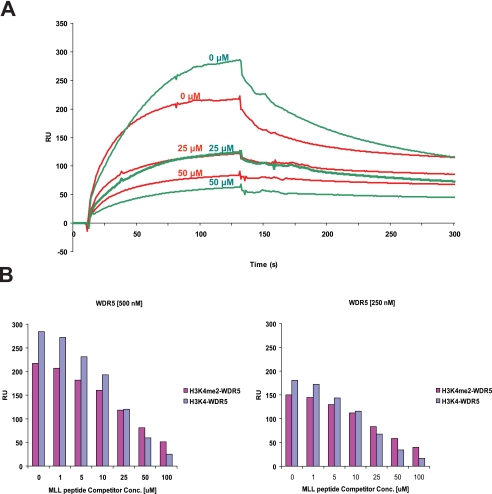
FIGURE 5.
Histone binding properties of WDR5. A, the binding of WDR5 for unmodified and di-methylated histone H3 peptide was measured in the presence of MLL competitor peptides. Representative sensorgrams are shown in red for di-methylated histone H3 and green for unmodified histone H3 binding in the presence of MLL peptides. Unmodified or di-methylated histone H3 peptide was immobilized, and WDR5 (500 nm) was injected with the MLL peptides (0, 25, 50 μm). B, the amount of WDR5 bound to the peptides is shown in a bar graph (di-methylated histone H3: magenta color, unmodified H3: purple and gray blue color). For binding experiments, WDR5 at two different concentrations (250, 500 nm) were injected with the MLL peptides (0, 1, 5, 10, 25, 50, 100 μm) (supplemental Fig. S3).