Abstract
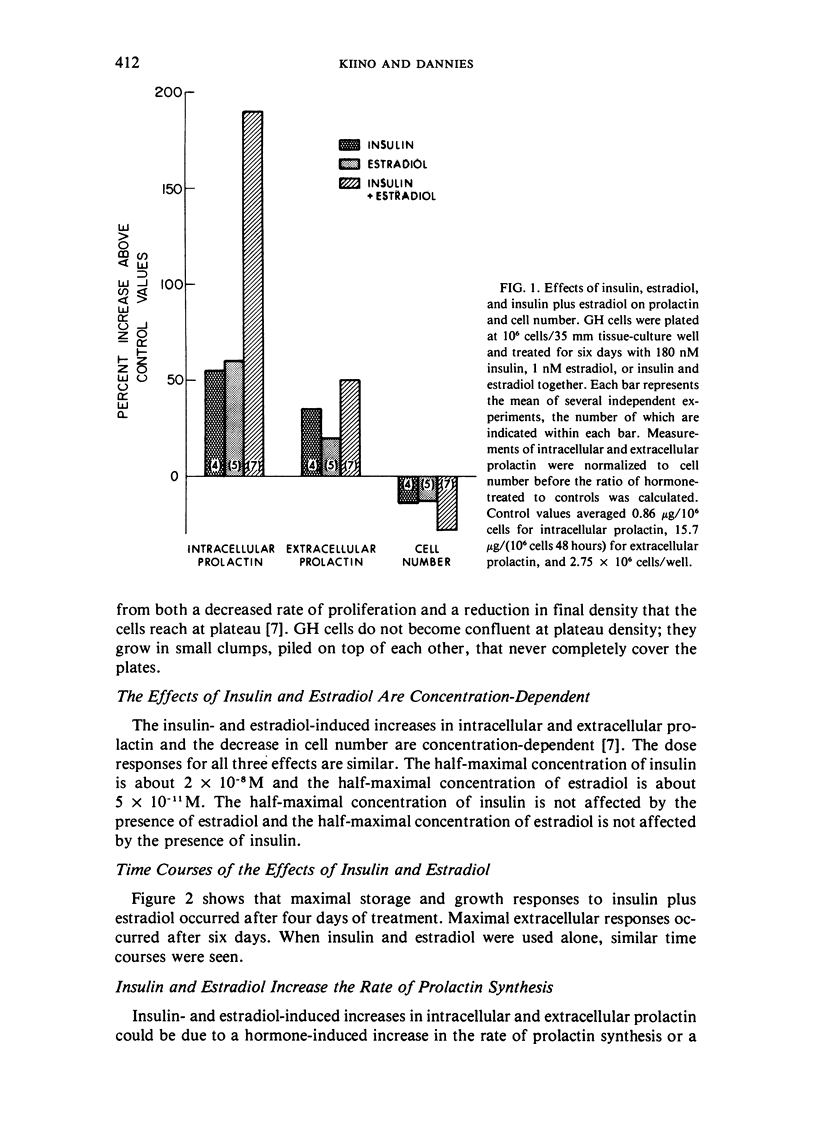
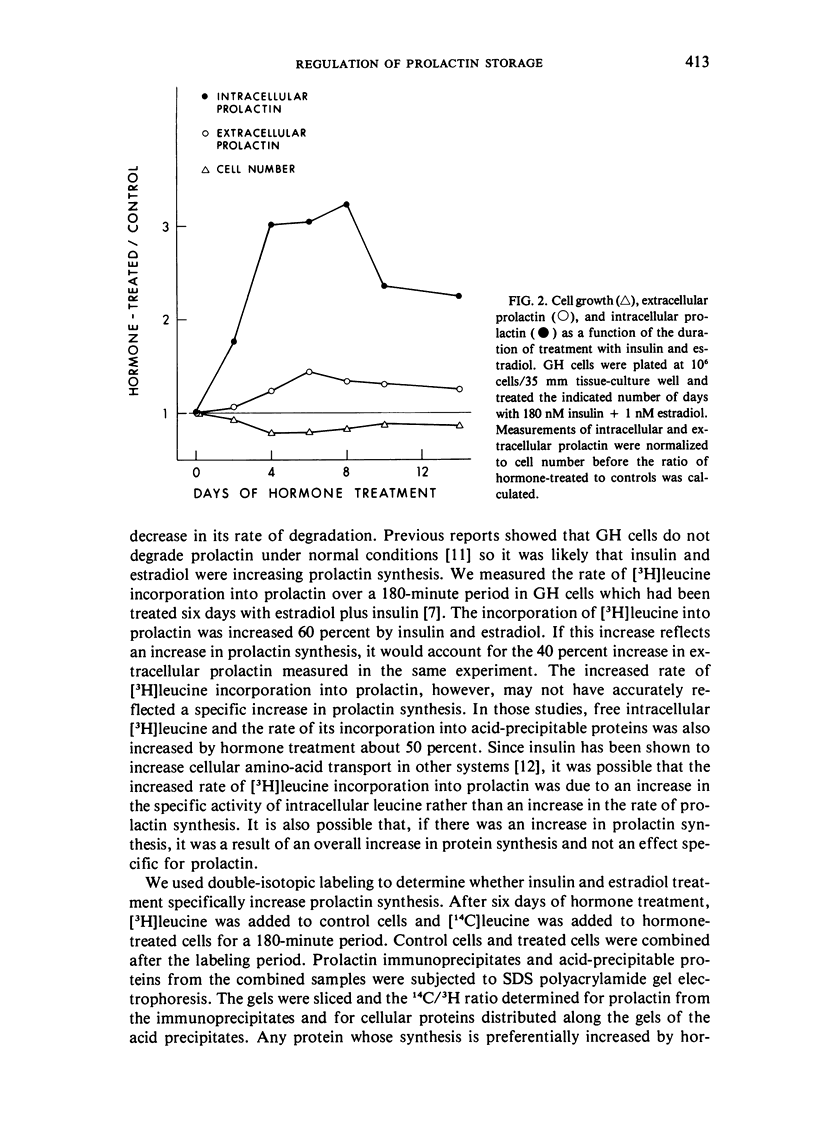
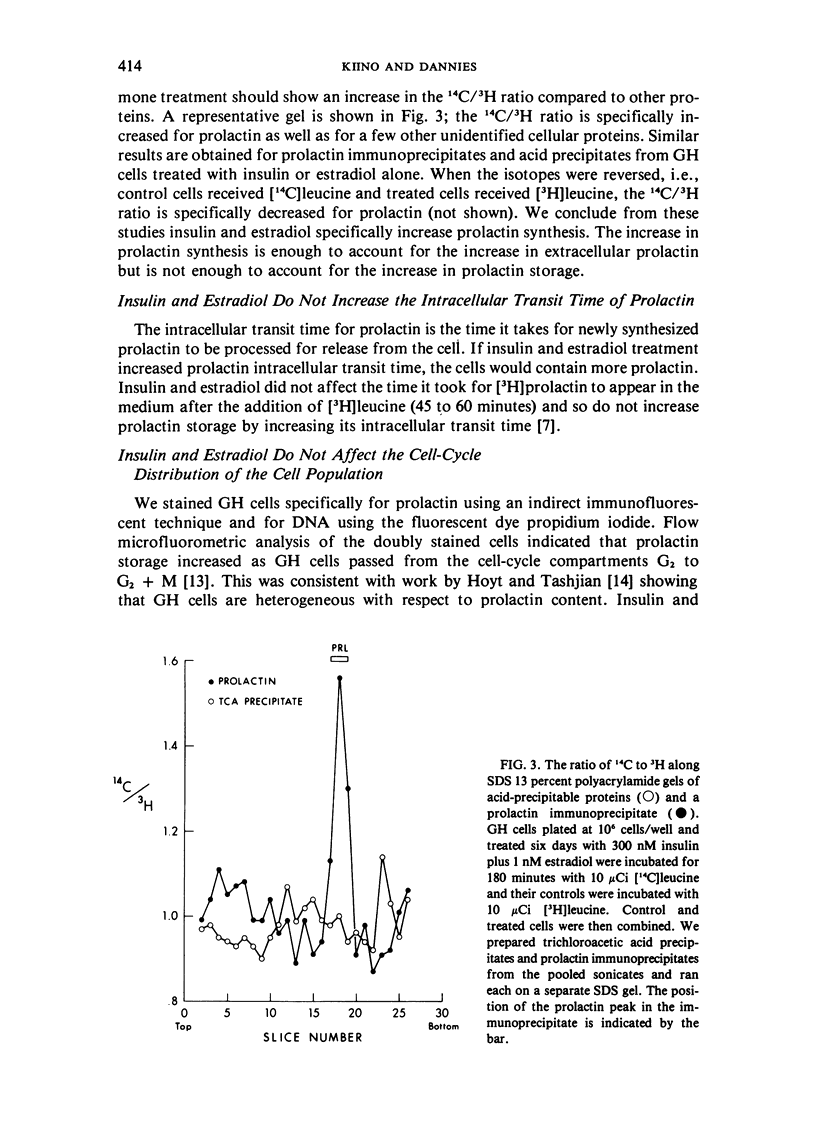
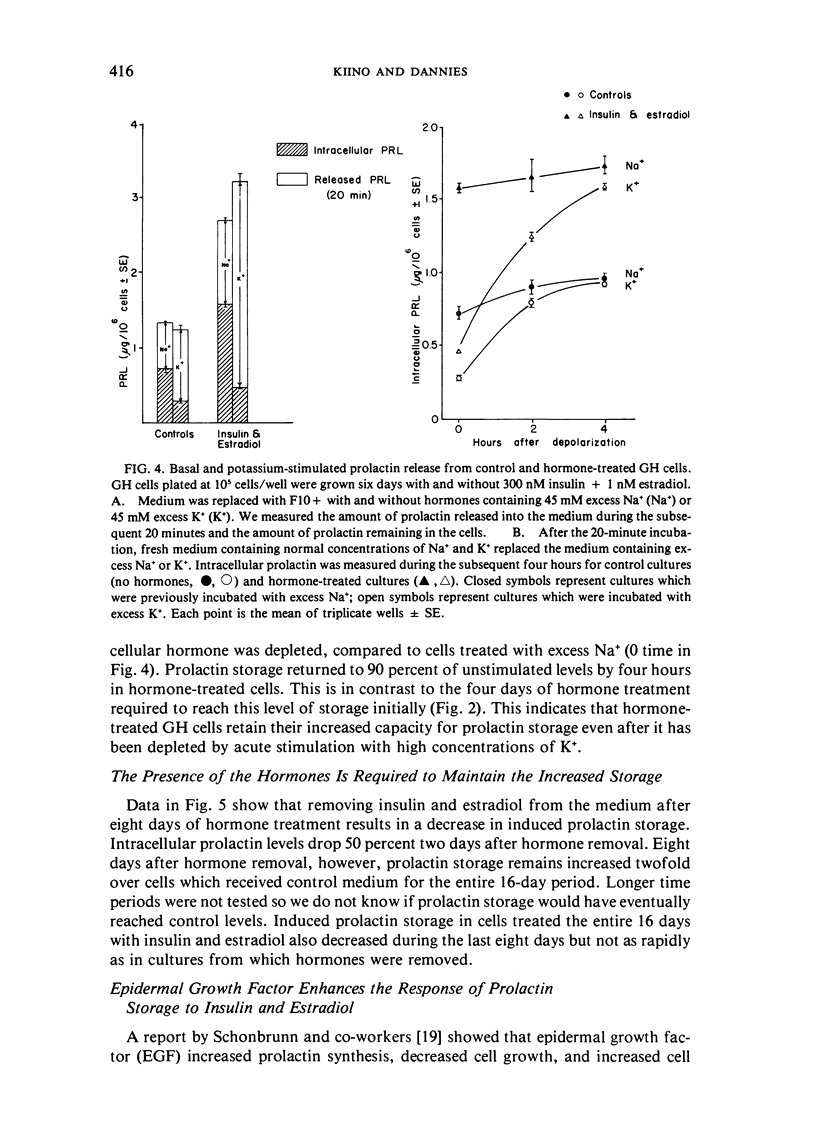
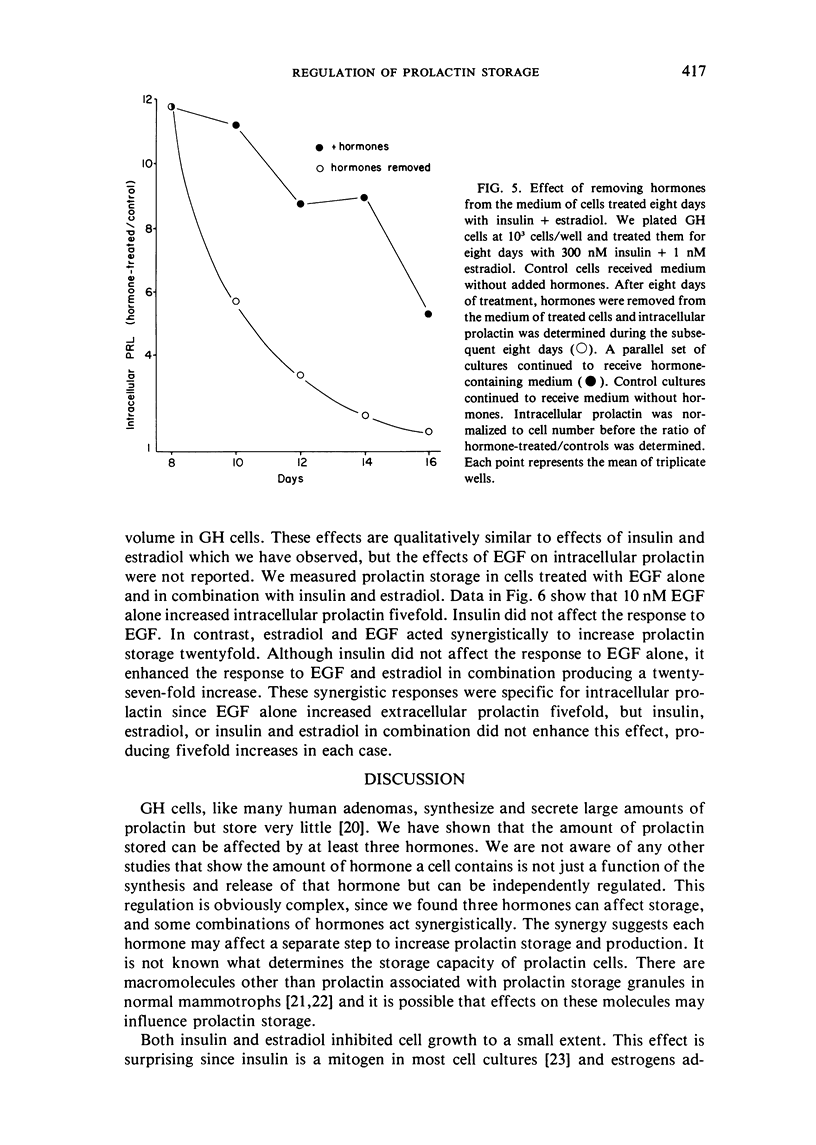
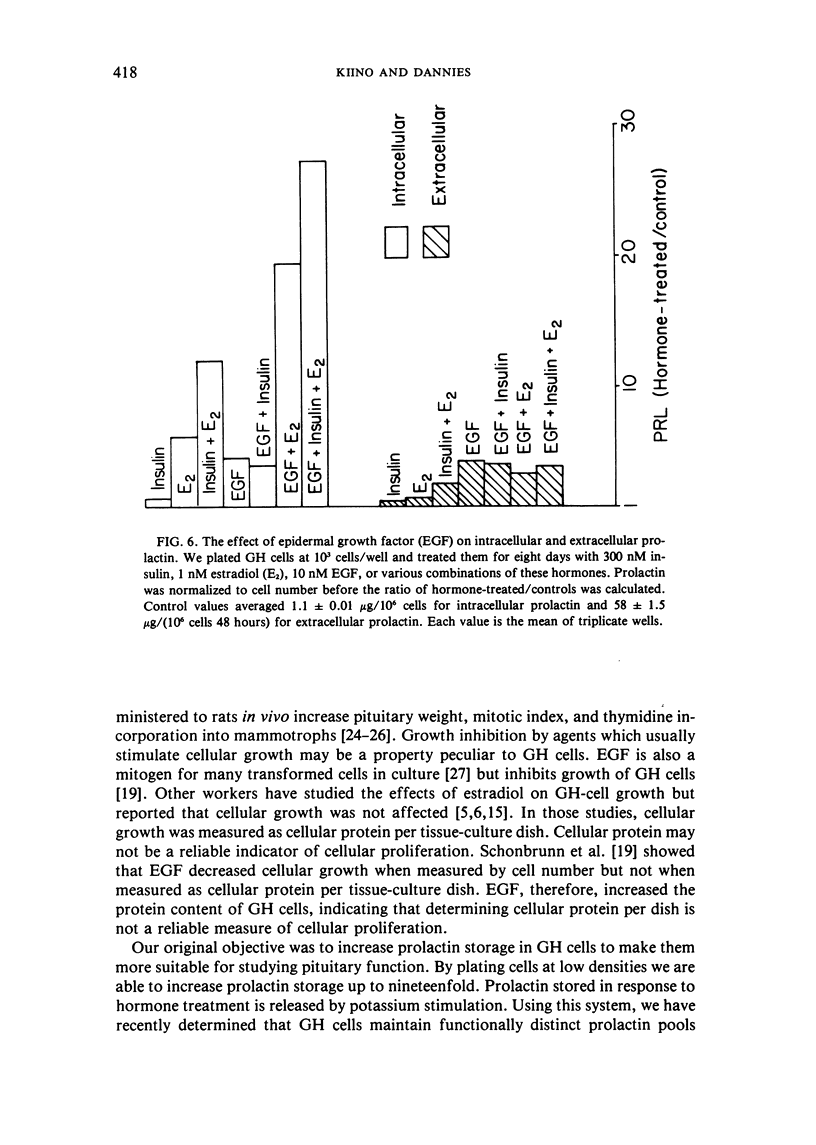
GH4C1 cells (GH cells) are a clonal strain of rat pituitary tumor cells which secrete prolactin. GH cells have been used to study hormone secretion, but they store relatively little prolactin compared to normal prolactin-secreting cells. They are not suitable, therefore, for studying some aspects of pituitary function. We have found that the amount of prolactin GH cells store can be regulated. When GH cells were plated at 10(6) cells/well and treated for six days with 180 nM insulin or 1 nM estradiol, there was a 60 percent increase in prolactin storage compared to control cells. Insulin and estradiol in combination acted synergistically to cause a 190 percent increase in prolactin storage. In contrast, they were additive in increasing extracellular prolactin; there was a 40 percent increase in extracellular prolactin after insulin, a 20 percent increase after estradiol, and a 50 percent increase after insulin plus estradiol. The increases in prolactin storage were always greater than the increases in extracellular prolactin. The increases in prolactin storage were dose-dependent and reached maximal levels after four days of treatment with 180 nM insulin plus 1 nM estradiol. Reducing the plating density to 10(3) cells/well increased the response to insulin and estradiol to nineteenfold. Epidermal growth factor (10 nM) acted synergistically with estradiol and insulin in combination to increase prolactin storage 27-fold. The insulin- and estradiol-induced increase in extracellular prolactin was caused by a specific increase in the rate of prolactin synthesis. The fractional increase in prolactin storage above the increase in prolactin production could not be explained by an increase in prolactin synthesis, an increase in intracellular transit time, or a change in the cell-cycle distribution of the population. Hormone storage can, therefore, be regulated independently from other processes which control hormone production. The prolactin stored in response to insulin and estradiol was releasable by potassium depolarization. Following depletion of intracellular prolactin by depolarization, the cells retained their increased capacity for prolactin storage. The ability to increase prolactin storage will make GH cells a more useful system in which to study pituitary function.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Clausen O. P., Gautvik K. M., Haug E. Effects of cortisol, 17beta-estradiol and thyroliberin on prolactin and growth hormone production, cell growth and cell cycle distribution in cultured rat pituitary tumour cells. J Cell Physiol. 1978 Feb;94(2):205–214. doi: 10.1002/jcp.1040940210. [DOI] [PubMed] [Google Scholar]
- Corenblum B., Kovacs K., Penz G., Ezrin C. The effects of estrogen on prolactin cells of the male rat pituitary. An immunocytologic and autoradiographic study. Endocr Res Commun. 1980;7(3):137–144. doi: 10.3109/07435808009065967. [DOI] [PubMed] [Google Scholar]
- Dannies P. S., Tashjian A. R., Jr Effects of thyrotropin-releasing hormone and hydrocortisone on synthesis and degradation of prolactin in a rat pituitary cell strain. J Biol Chem. 1973 Sep 10;248(17):6174–6179. [PubMed] [Google Scholar]
- Haug E., Gautvik K. M. Effects of sex steroids on prolactin secreting rat pituitary cells in culture. Endocrinology. 1976 Dec;99(6):1482–1489. doi: 10.1210/endo-99-6-1482. [DOI] [PubMed] [Google Scholar]
- Haug E., Tjernshaugen H., Gautvik K. M. Variations in prolactin and growth hormone production during cellular growth in clonal strains of rat pituitary cells. J Cell Physiol. 1977 Apr;91(1):15–29. doi: 10.1002/jcp.1040910103. [DOI] [PubMed] [Google Scholar]
- Holley R. W. Control of growth of mammalian cells in cell culture. Nature. 1975 Dec 11;258(5535):487–490. doi: 10.1038/258487a0. [DOI] [PubMed] [Google Scholar]
- Hoyt R. F., Jr, Tashjian A. H., Jr Immunocytochemical analysis of prolactin production by monolayer cultures of GH3 rat anterior pituitary tumor cells: II. Variation in prolactin content of individual cell colonies, and dynamics of stimulation with thyrotropin-releasing hormone (TRH). Anat Rec. 1980 Jun;197(2):163–181. doi: 10.1002/ar.1091970206. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Flier J. S., Grunfeld C., Harmon J. T., Harrison L. C., Karlsson F. A., Kasuga M., King G. L., Lang U. C. Insulin receptors, receptor antibodies, and the mechanism of insulin action. Recent Prog Horm Res. 1981;37:477–538. doi: 10.1016/b978-0-12-571137-1.50015-3. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kiino D. R., Burger D. E., Dannies P. S. Prolactin storage in a clonal strain of rat pituitary tumor cells is cell-cycle dependent. J Cell Biol. 1982 May;93(2):459–462. doi: 10.1083/jcb.93.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiino D. R., Dannies P. S. Insulin and 17 beta-estradiol increase the intracellular prolactin content of GH4C1 cells. Endocrinology. 1981 Oct;109(4):1264–1269. doi: 10.1210/endo-109-4-1264. [DOI] [PubMed] [Google Scholar]
- Lloyd H. M., Meares J. D., Jacobi J. Early effects of stilboestrol on growth hormone and prolactin secretion and on pituitary mitotic activity in the male rat. J Endocrinol. 1973 Aug;58(2):227–231. doi: 10.1677/joe.0.0580227. [DOI] [PubMed] [Google Scholar]
- Ostlund R. E., Jr, Leung J. T., Hajek S. V., Winokur T., Melman M. Acute stimulated hormone release from cultured GH3 pituitary cells. Endocrinology. 1978 Oct;103(4):1245–1252. doi: 10.1210/endo-103-4-1245. [DOI] [PubMed] [Google Scholar]
- Schonbrunn A., Krasnoff M., Westendorf J. M., Tashjian A. H., Jr Epidermal growth factor and thyrotropin-releasing hormone act similarly on a clonal pituitary cell strain. Modulation of hormone production and inhbition of cell proliferation. J Cell Biol. 1980 Jun;85(3):786–797. doi: 10.1083/jcb.85.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaby F., Farquhar M. G. Characterization of rat somatotroph and mammotroph secretory granules. Presence of sulfated molecules. Mol Cell Endocrinol. 1980 Apr;18(1):33–48. doi: 10.1016/0303-7207(80)90005-2. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Electrical behaviour in a line of anterior pituitary cells (GH cells) and the influence of the hypothalamic peptide, thyrotrophin releasing factor. Neuroscience. 1980;5(2):421–431. doi: 10.1016/0306-4522(80)90117-7. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Bancroft F. C., Levine L. Production of both prolactin and growth hormone by clonal strains of rat pituitary tumor cells. Differential effects of hydrocortisone and tissue extracts. J Cell Biol. 1970 Oct;47(1):61–70. doi: 10.1083/jcb.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Lomedico M. E., Maina D. Role of calcium in the thyrotropin-releasing hormone-stimulated release of prolactin from pituitary cells in culture. Biochem Biophys Res Commun. 1978 Apr 14;81(3):798–806. doi: 10.1016/0006-291x(78)91422-5. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Zanini A., Giannattasio G., Nussdorfer G., Margolis R. K., Margolis R. U., Meldolesi J. Molecular organization of prolactin granules. II. Characterization of glycosaminoglycans and glycoproteins of the bovine prolactin matrix. J Cell Biol. 1980 Jul;86(1):260–272. doi: 10.1083/jcb.86.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]


