Abstract
Objective
Low dose stimulation (LS) is emerging as an alternative regime in assisted reproductive technology (ART). This study aimed to compare the cost-effectiveness of LS to the high dose GnRH antagonist (Atg) regime.
Methods
An observational prospective study conducted at an academic infertility unit from January to June 2007. Outcome measures included the numbers of follicles, oocytes and embryos, morphological quality of oocytes and embryos, clinical pregnancy (PR) and complication rate.
Result
Ninety five first attempt ICSI cycles consisting of 54 LS and 41 Atg were analyzed. Subjects in both groups had comparable sociodemographics and reproductive characteristics. LS generated significantly fewer follicles, total oocytes, mature oocytes (all p < 0.0005) and immature oocytes (p = 0.009) than Atg but the number of excellent quality oocytes was similar. Significantly fewer embryos were available in LS although the proportion of usable embryos was higher, 83.2% vs. 67.0% for Atg. Mean embryos per transfer was 2.0 ± 1.1 vs. 2.6 ± 1.0 (p = 0.02) for a clinical PR per transfer of 43.2% vs. 50.0% for LS and Atg respectively. LS regime had a shorter gonadotrophin administration period with resultant COH cost one third of the Atg protocol (both, p < 0.0005). The cost per live birth per started cycle worked out to be USD 13,200 and 24,900 for LS and Atg respectively. Furthermore, LS had fewer incidences of OHSS compared to the Atg regime, 3.7% vs. 12.2%.
Conclusion
LS cost benefits included lower amounts of gonadotrophin used and fewer injections. It is a viable alternative regime in producing comparable clinical PR at lower cost and less complication in ART.
Keywords: Ovarian stimulation, Low dose stimulation, GnRH antagonist, ICSI, ART
Introduction
Assisted reproductive technology (ART) is an expensive and protracted process with an uncertain outcome for those seeking infertility treatments. The most important consideration is financial resources as most medication and treatment cost in developing countries are not government subsidized or covered by insurance [1]. Complex, costly and lengthy ovarian stimulation protocols increases the financial demand on the patient as well as certain complications and risks [2, 3].
Natural cycle IVF offers several advantages over controlled ovarian stimulation (COH), with no risk of ovarian stimulation and multi-fetal gestation. But the efficacy of natural cycle is limited, mainly due to low number of oocytes and high cancellation rates caused by the occurrence of spontaneous LH surges [4–7]. Low dose stimulation (LS) offers the same advantages as natural cycle IVF but had a higher efficacy by recruiting one dominant follicle and several secondary follicles that spontaneously develop in a natural cycle [8, 9].
However, there were reservations that the low dose approach may be counter productive as most couples could only afford one, at most two cycles. Hence an aggressive approach may better serve them by maximizing success in one cycle. Therefore, the purpose of this study was to assess whether LS may be a cost-beneficial alternative to the high dose GnRH antagonist (Atg) regime [10–12].
Materials and methods
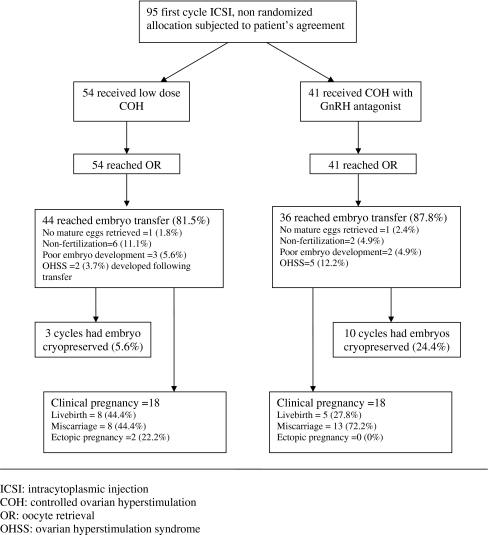
A prospective study involving 95 couples undergoing intracytoplasmic sperm injection (ICSI) was conducted at a tertiary government hospital from 1st January to 30th June 2007. Ethical consideration demanded that all couples be offered LS as a choice with the benefits and disadvantages carefully discussed. Patients who decided not to participate in the LS study underwent the Atg protocol. Only the first attempt was included in the analysis. The study design is shown in Fig. 1.
Fig. 1.
Study design
All couples must be married in accordance with existing local laws. Basic infertility evaluation included semen parameters, ovulation assessment, ultrasonography of female pelvic anatomy and tubal patency via laparoscopy or hysterosalpingography. Azoospermic cases were excluded as no donor program was allowed. The subjects were pooled into major infertility groups based on primary indications. Ovarian pathology was determined by transvaginal ultrasound. Polycystic ovarian syndrome was concluded on the presence of a thickened capsule or necklace ring on ultrasound, hirsutism/acne and/or high serum testosterone. Normal ovarian reserve was based ultrasound measurements [13] and day three serum FSH and LH [14]. All subjects except two had FSH below 10 UI/L. One subject in each study group had high FSH (Atg 21.0 vs. MS 15.9 IU/L) but with a FSH/LH ratio below 2.6 and was included in the study [15]. The extent of endometriosis was determined by diagnostic laparoscopy six to 9 months prior and scored according to the AFS guidelines [16]. Only mild to moderate stages were included in the study as no subject demonstrated severe endometriosis. Patients with male factor or unexplained subfertility had undergone treatment with intrauterine insemination for three to six cycles before initiating ART. Male factor infertility was based on semen diagnosis of less than 5 × 106/mL motile sperms.
Day 2 basal scan was performed for all patients at cycle commencement. Patients were excluded from the study if endometrial thickness was >4 mm and if there was presence of any cyst. In the LS group, the stimulation was started with clomiphene citrate 100 to 150 mg daily from 2nd to 6th day followed by low dose recombinant FSH (75 to 150 IU) until a leading follicle of 18 mm was observed. Ovulation was triggered by 10,000 IU of human chorionic gonadotrophin (hCG). No GnRH antagonist was used in this group of patient. In the Atg group, rFSH (225 to 450 IU) was used for ovulation induction from 2nd day of the cycle till ovulation triggering. When a lead follicle with a mean diameter of at least 13 mm was observed, daily injections of 0.25 mg Atg (Cetrorelix) were started and continued up to the day of hCG injection. There was no prior GnRH agonist down-regulation of the pituitary–ovarian axis in LS and Atg groups.
Ultrasound monitoring began on cycle day 6 and repeated every other day. Follicular size was measured in three perpendicular planes and the mean value was taken. Ovulation triggering was achieved by subcutaneous injection of 10,000 IU of HCG when a follicle with of 18 mm in diameter or larger was observed. Transvaginal ultrasound-guided oocyte retrieval was performed 33–36 h after ovulation triggering, under sedation (Midazolam 5 mg and Pethidine 100 mg). Oocyte retrieval was carried out transvaginally using an aspiration needle (Cook, Australia). All procedures [17] were carried out in the conventional manner using GIII series media (USA).
Washed sperms from fresh ejaculates after 3–7 days abstinence were obtained on the morning of oocyte retrieval. Semen analysis was performed according to WHO criteria [18]. Teratozoospermia was the major defect in the male subjects, occurring in 79% with or without concomitant oligozoospermia and astenozoospermia. It was the general consensus that ICSI should be carried out for all cases. Oocytes were incubated for 3–6 h and oocyte assessment performed at the time of ICSI [19]. ICSI was performed with a semi-automated micromanipulator (Narashige, Japan) with mature oocytes injected with a single motile sperm in commercial PVP (Fertipro, Belgium). Normal motile sperms were selected for ICSI whenever possible. Abnormal (borderline) sperm ratio in ICSI was 36.4% vs. 41.1% for LS and Atg group respectively.
Fertilization was observed 16–18 h after ICSI and fertilization rate was based on the number of zygotes with two or more pronuclei over MII eggs. Cleavage rate was defined as the number of two cell embryos over fertilized eggs. Blastomere nuclearity was assessed at 44–46 h post ICSI [20]. Selection of embryos for transfer was according to the less critical Bournhall guideline [21] taking into account blastomeres nuclearity. Preferentially, embryos with multinuclear blastomeres were not transferred unless there were no available embryos. Then only those with the fewest multinuclear blastomeres were chosen. All embryo transfers were performed 72–76 h after oocyte retrieval. The number of embryos transferred was according to ASRM guidelines [22].
The luteal phase was supported by hCG 5,000 IU on day 5, 8, 11 and 14 post oocyte retrieval with either oral or vaginal progesterone capsules or both, until 8 weeks gestation. The cancellation rate was defined as initiated cycles that did not reach the oocyte retrieval stage. Clinical pregnancy was defined as the ultrasound visualization of a distinct intrauterine gestational sac and embryonic cardiac activity at a minimum of 5 weeks after oocyte retrieval. Implantation rate was defined as the number of gestational sac per embryo transferred.
Data analysis
The data were analyzed using the SPSS statistical software package. The differences between treatment groups were assessed by appropriate tests with significance set at the 0.05 level.
Result
Ninety-five first attempt ICSI cycles were included in the study. Patient characteristics and indication are shown in Table 1. Majority of the patients were ethnic Malays (83.2%). Fifty four and 41 patients underwent COH with LS and Atg regime respectively. There were more Malays in the LS group than the Atg group (p = 0.001), a distribution that could be attributed to economic disparity as financial considerations were the primary reason for accepting the LS protocol. There were no racially discordant couples in this small group. Over a quarter of the patients had severe male factor (26.3%) and unexplained infertility (25.3%). The other major infertility factors were endometriosis (14.7%), tubal (14.7%), ovarian (10.5%) and combined factors (8.4%). Subjects in both groups were comparable in age, all aspect of infertility diagnosis, duration of infertility and semen parameters. The overall clinical pregnancy rate (PR) was 38.9% per started cycle.
Table 1.
Sociodemographic and reproductive characteristics of subjects
| Characteristics (N = 95) | Low dose (N = 54) | GnRH antagonist (N = 41) | p valuea |
|---|---|---|---|
| N (%) | |||
| Ethnicity (female) | 0.001a | ||
| Malay | 50 (92.6) | 29 (70.7) | |
| Chinese | 2 (3.7) | 13 (29.3) | |
| Indian | 2 (3.7) | – | |
| Age distribution (female, years) | NS | ||
| =/<25 | 1 (1.8) | 0 (0) | |
| 26 to 30 | 12 (22.2) | 8 (19.5) | |
| 31 to 35 | 22 (40.7) | 16 (39.0) | |
| 36 to 40 | 14 (25.9) | 9 (21.9) | |
| =/>41 | 5 (9.2) | 8 (19.5) | |
| Primary sub-fertility | 97 (80.8) | 65 (82.3) | NS |
| Presenting infertility factors | NS | ||
| Tubal | 9 (16.7) | 5 (12.2) | |
| Male factor | 11 (20.4) | 14 (34.1) | |
| Endometriosis | 10 (18.5) | 4 (9.8) | |
| Idiopathic | 14 (25.9) | 10 (24.4) | |
| PCOS/ovarian | 7 (13.0) | 3 (7.3) | |
| Combination | 3 (5.6) | 5 (12.2) | |
| Mean±SD | |||
| Age of female subjects (years) | 34.0 ± 4.6 | 35.4 ± 4.9 | NS |
| Age of male subjects (years) | 37.0 ± 4.8 | 39.6 ± 7.3 | 0.048 |
| Duration of infertility (years) | 6.0 ± 4.2 | 6.1 ± 3.1 | NS |
| Day 3 serum FSH (IU/L) | 7.0 ± 4.1 | 7.8 ± 4.9 | NS |
| Day 3 serum LH (IU/L) | 5.0 ± 3.2 | 5.6 ± 3.8 | NS |
| Semen parameters | |||
| Density (×106 per mL) | 75.8 ± 69.4 | 60.8 ± 68.7 | NS |
| Volume (per mL) | 2.3 ± 0.6 | 2.5 ± 1.4 | NS |
| Forward progression (%) | 45.4 ± 20.2 | 39.7 ± 21.3 | NS |
| Normal morphology, WHO (%) | 14.6 ± 11.6 | 14.3 ± 10.5 | NS |
NS Not significant
aCategorical and continuous variables were analysed by Pearson Chi-square and t test respectively. There were no racially discordant couples in the study.
A total of 281 and 463 oocytes were recovered from LS and Atg group respectively with a similar proportion of immature oocytes, 18.9% vs. 18.1%. The LS group had significantly fewer follicles, total oocytes, mature oocytes (all p < 0.0005) and immature oocytes (p = 0.009) than the Atg group (Table 2). Nevertheless number of excellent quality oocytes in both groups was similar.
Table 2.
Characteristics of ART cycles
| Characteristics (N = 95) | Low dose (N = 54) | GnRH antagonist (N = 41) | p valuea |
|---|---|---|---|
| N (%) | |||
| No. embryo transfer cycles | 44 (81.5) | 36 (87.8) | NS |
| Clinical pregnancy rate per embryo transfer | 19 (43.2) | 18 (50.0) | NS |
| Clinical pregnancy rate per cycle started | 19 (35.2) | 18 (43.9) | NS |
| No. mature oocytes per retrieval | 0.031 | ||
| 1–2 oocytes | 17 (31.5) | 4 (9.8) | |
| 3–6 oocytes | 27 (50.0) | 13 (31.7) | |
| >6 oocytes | 9 (16.7) | 23 (56.1) | |
| No. embryos per transfer | 0.052 | ||
| 1 embryo | 17 (38.6) | 7 (19.4) | |
| 2 embryos | 12 (27.3) | 6 (16.7) | |
| 3 embryos | 9 (20.5) | 18 (50.0) | |
| 4 embryos | 6 (13.6) | 5 (13.9) | |
| Mean±SD | |||
| Oocytes | |||
| No. follicles observed (>12 mm) | 7.3 ± 5.3 | 13.1 ± 7.2 | 0.0005 |
| No. oocytes retrieved | 5.2 ± 4.9 | 11.9 ± 8.5 | 0.0005 |
| No. mature oocytes (MII) | 4.2 ± 3.8 | 9.5 ± 6.4 | 0.0005 |
| Excellent morphology | 1.4 ± 2.2 | 1.0 ± 1.9 | NS |
| Good morphology | 2.2 ± 2.0 | 6.6 ± 6.2 | 0.0005 |
| Fair morphology | 0.3 ± 0.7 | 1.5 ± 2.0 | 0.0005 |
| Poor morphology | 0.3 ± 0.9 | 0.3 ± 0.8 | NS |
| No. immature oocytes (MI and GV) | 0.9 ± 1.5 | 2.0 ± 2.4 | 0.009 |
| No. degenerated/defective oocytes | 0.2 ± 0.5 | 0.4 ± 0.9 | NS |
| Fertilization and cleavage (2 cell stage) | |||
| No. oocytes fertilized | 2.6 ± 2.7 | 6.0 ± 4.0 | 0.0005 |
| Fertilization rate (%) | 59.3 ± 30.9 | 63.5 ± 24.4 | NS |
| Cleavage rate (%) | 94.1 ± 19.8 | 89.4 ± 25.0 | NS |
| Embryos | |||
| No. embryos developed | 2.5 ± 2.5 | 5.7 ± 4.2 | 0.0005 |
| Excellent morphology | 0.5 ± 0.8 | 1.3 ± 1.7 | 0.011 |
| Good morphology | 1.2 ± 1.9 | 2.2 ± 2.1 | 0.021 |
| Moderate to fair morphology | 0.5 ± 0.8 | 1.7 ± 2.4 | 0.002 |
| Poor morphology | 0.5 ± 0.8 | 0.8 ± 1.6 | NS |
| No. embryos transferred | 2.0 ± 1.1 | 2.6 ± 1.0 | 0.020 |
| No. embryos cryopreserved | 5.7 ± 1.5 | 5.8 ± 2.1 | NS |
| Duration of COH (days) | 12.2 ± 2.4 | 11.4 ± 1.7 | NS |
| Duration if gonadotrophin injection (days) | 7.4 ± 2.3 | 11.2 ± 1.8 | 0.0005 |
| Endometrial thickness (mm) | 10.4 ± 1.9 | 11.3 ± 1.7 | 0.026 |
| Amount of gonadotrophin used (IU) | 974 ± 415 | 3,007 ± 805 | 0.0005 |
| Cost of ovarian stimulation (USD) | 443 ± 188 | 1,517 ± 367 | 0.0005 |
NS Not significant
aCategorical and continuous variables were analysed by Pearson Chi-square and t test respectively.
In all, there were 152 vs. 227 cleaved embryos for LS and Atg respectively. Although the LS group had less embryos (p < 0.0005) and hence fewer excellent and good morphology embryos (p = 0.011 and 0.021), there was a higher proportion of usable embryos, 83.2% vs. 67.0% for Atg group. Less embryos were transferred per cycle (p = 0.02) in LS group compared to Atg (Table 2).
LS and Atg group had a similar distribution of multinuclear embryos. Overall, 8% of embryos had all mononuclear blastomeres, 9% having a mixture of mono and non-nucleated blastomeres and 16% were all non-nucleated while half (49%) had one or more bi/multinucleated blastomeres and 18% were embryos with all multinucleated blastomeres.
Clinical PR per transfer was 43.2% and 50.0% whereas implantation rate was 20.6% (19 gestational sacs per 92 embryos) and 20.2% (19 gestational sacs per 94 embryos) respectively for LS and Atg group. There were eight and five live birth in LS and Atg group respectively for a combined take home baby rate of 16.2% per embryo transfer and 13.7% per started cycle. All were singletons except for a pair of twin in the Atg group for an overall multiple pregnancy rates of 7.7%.
Atg group had significantly more freezing cycles compared to the LS protocol (p = 0.001). Except for a thicker endometrium in the Atg group (p = 0.026), other cycle parameters were similar in both groups. There was no cancellation with all cycles reaching oocyte retrieval followed by 80 transfers (84.2%). No embryo transfer was carried out 15 cycles due to fertilization failure (8.4%) or arrested embryonic development (5.3%). Majority of them occurred in subjects with small number of retrieved oocytes (one to two oocytes, 80%). There were two cases of mild OHSS in LS (3.7%) compared to five cases in Atg group (12.2%) whereby embryo transfers were carried out without negative consequences (Fig. 1).
Overall, the amount of gonadotrophin used was two thirds lower and the period of gonadotrophin administration was significantly shorter in LS group ensuing in lower cost of COH (all, p < 0.0005) and a more comfortable treatment process (Table 2). The amount of gonadotrophin used and cost of COH tended to increase with age but not significantly so (Table 3). The number of oocytes retrieved and mature oocytes however did diminished significantly with increasing age of subjects. But it was still possible to obtain mature oocytes from older women (=/>41 years old) using the LS regime.
Table 3.
Gonadotrophin, cost and oocyte recovery by stimulation regime and age group
| Age category (years) | No. subjects | Amount of gonadotrophin used (IU) | Cost of ovarian stimulation (USD) | No. oocytes retrieved | No. mature oocytes (MII) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LS | Atg | LS | Atg | LS | Atg | LS | Atg | LS | Atg | |
| Mean±SD | ||||||||||
| </=30 | 13 | 8 | 1,073 ± 382 | 2,829 ± 792 | 488 ± 174 | 1,447 ± 366 | 9.0 ± 8.2 | 10.8 ± 7.2 | 7.0 ± 6.3 | 8.8 ± 7.0 |
| 31–35 | 22 | 16 | 1,014 ± 467 | 2,866 ± 730 | 461 ± 212 | 1,448 ± 331 | 3.9 ± 2.0 | 14.6 ± 9.7 | 3.3 ± 1.8 | 11.2 ± 6.0 |
| 36–40 | 14 | 9 | 789 ± 332 | 3,024 ± 592 | 359 ± 151 | 1,522 ± 269 | 3.9 ± 2.1 | 14.7 ± 7.3 | 3.3 ± 2.0 | 12.0 ± 6.2 |
| =/>41 | 5 | 8 | 1,065 ± 405 | 3,450 ± 1,108 | 484 ± 184 | 1,720 ± 504 | 4.8 ± 5.2 | 4.8 ± 3.8 | 3.8 ± 2.9 | 4.0 ± 4.2 |
LS: Low dose regime. ANOVA: no. oocytes retrieved (p = 0.013), no. mature oocytes (p = 0.021)
Atg: GnRH antagonist regime. ANOVA: no. oocytes retrieved (p = 0.032), no. mature oocytes (p = 0.031)
Based on total treatment expense and calculated on the basis of the numbers needed to treat, the cost of one live birth in the LS group and Atg group per started cycle worked out to be USD 13,200 and 24,900 respectively.
Discussion
The study was limited by a small patient population with dissimilar racial distribution and mixed infertility factors. Only the first COH attempt was taken into analysis to avoid management bias. All subjects proceeded to oocyte retrieval as cancellation was avoided whenever possible. Conservative management of these poor responders (22%, one to two oocytes) had in effect reduced the overall PR. The advantage of using ICSI in all cycles may have possibly allowed a better comparison between the COH protocols by overcoming minor sperm anomalies that could have impeded fertilization.
The strategy of administering low doses of FSH in the late follicular phase resulted in fewer oocytes being available but the smaller cohort of growing follicles may improve oocyte quality and the capacity to be fertilized. Recent studies have shown while conventional COH resulted in more embryos than the mild approach, the number of chromosomally normal embryos were similar [23]. In concurrence, the study showed that the LS regime produced a similar number of excellent quality oocytes compared to the Atg regime.
Reduced stimulation may also avoid the adverse effects of hyperstimulation on corpus luteum function and endometrial receptivity [8]. Sustaining the latter hypothesis, we observed a similar implantation rate in both treatment groups even though the number of excellent morphology embryos was significantly lower in the LS group. Additionally, the miscarriage rate was also lower in the LS group. This small study is unable to provide more information on the causes and rate of miscarriage. It is an interesting aspect of stimulation protocols that would require a larger and more complex study.
The number of available embryos in LS was sufficient because of the present trend of transferring fewer embryos. Several studies have shown this to be effective in reducing the risk of multi-fetal gestation [24]. Current consensus deemed a transfer of large numbers of embryos to increase “success rates” per IVF cycle is not justifiable. But for the majority of infertile patients, twins represent a favourable and cost-effective treatment outcome [25]. Such a view is particularly strong in Asian societies where fertility is highly valued. Twin pregnancies are viewed as auspicious and very much desired. Two or more embryos per transfer were often requested by patients. Due to availability, the number of embryos transferred was higher in the Atg group. This may have biased the PR rate toward Atg regime.
The study only included fresh embryos transfers as endpoints. There are 13 cycles with cryopreserved embryos that may impact the final outcome but so far, studies have shown that the increase in pregnancy rates with frozen embryos transfer was modest [26]. With a low cost stimulation regime, it may be more cost beneficial to carry out a consecutive fresh cycle rather than a thaw cycle in particular if treatment costs are affordable.
In this study, the cost of COH per cycle was three fold higher in the Atg group compared to the LS protocol. Therefore, the LS protocol could be an alternative for ART patients with financial constraint. The reduced duration of exogenous FSH administration shall significantly reduce monitoring and increase patients’ comfort. Short-term complications such as OHSS and long-term risks may be reduced. No resting cycle was necessary after a failed cycle, and treatment can be performed in consecutive cycles. Therefore, the cumulative pregnancy rates would be higher in the long run [27].
The major inconvenience was the scheduling of oocyte retrieval procedures. As the LS protocol does not use GnRH antagonist to contain the LH surge, oocyte retrievals on weekends was unavoidable. But in a large unit, the work schedule may not pose a significant problem.
In conclusion, LS offered a low risk and patient friendly protocol, being associated with a lower risk of OHSS, less monitoring, a shorter duration of gonadotrophin injection, lower medication and overall treatment cost. The pregnancy rate of 43.2% per transfer in the LS group was lower than the Atg group but would still be considered acceptable. With its many advantages, LS is a feasible option for patients undergoing IVF.
References
- 1.Ombelet W, Campo R. Affordable IVF for developing countries. Reprod Biomed Online 2007;15:257–65. [DOI] [PubMed]
- 2.Ubaldi F, Rienzi L, Baroni E, et al. Hopes and facts about mild ovarian stimulation. Reprod Biomed Online 2007;14:675–81. [DOI] [PubMed]
- 3.Bouwmans CA, Lintsen BM, Eijkemans MJ, et al. A detailed cost analysis of in-vitro fertilization and intracytoplasmic sperm injection treatment. Fertil Steril 2008;89:331–41. [DOI] [PubMed]
- 4.Pelinck MJ, Hoek A, Simons AHM, Heineman MJ. Efficacy of natural cycle IVF: a review of the literature. Hum Reprod Update 2002;8:129–39. [DOI] [PubMed]
- 5.Rongieres-Bertrand C, Oliveness F, Righini C, Franchin R, et al. Revival of the natural cycle in in-vitro fertilization with the use of a new gonadotrophin-releasing hormone antagonist (Cetrorelix): a pilot study with minimal stimulation. Hum Reprod 1999;14:683–88. [DOI] [PubMed]
- 6.Pelinck MJ, Vogel NEA, Hoek A, et al. Minimal stimulation IVF with late follicular phase administration of the GnRH antagonist cetrorelix and concomitant substitution with recombinant FSH: a pilot study. Hum Reprod 2005;20:642–8. [DOI] [PubMed]
- 7.Meldrum DR, Rivier J, Garzo G, et al. Successful pregnancies with unstimulated cycle oocyte donation using an antagonist of gonadotrophin-releasing hormone. Fertil Steril 1994;61:556–7. [DOI] [PubMed]
- 8.Fauser BCJM, Devroey P, Yen SSC, et al. Minimal ovarian stimulation for IVF: appraisal of potential benefits and drawbacks. Hum Reprod 1999;14:2681–6. [DOI] [PubMed]
- 9.Williams SC, Gibbons WE, Muasher SJ, et al. Minimal ovarian hyperstimulation for in vitro fertilization using sequential clomiphine citrate and gonadotrophin with or without the addition of a gonadotropin-releasing hormone antagonist. Fertil Steril 2002;78:1068–72. [DOI] [PubMed]
- 10.Diedrich K, Diedrich C, Santos E, et al. Suppression of the endogenous luteinizing hormone surge by the gonadotrophin-releasing hormone antagonist Cetrorelix during ovarian stimulation. Hum Reprod 1994;9:788–91. [DOI] [PubMed]
- 11.Oliveness F, Franchin R, Bouchard P, et al. Scheduled administration of a gonadotrophin-releasing hormone antagonist (cetrorelix) on day 8 of in-vitro fertilization cycles: a pilot study. Hum Reprod 1995;10:1382–6. [PubMed]
- 12.Diedrich K, Felberbaum R. New approaches to ovarian stimulation. Hum Reprod 1998;13(Suppl.3):1–3. [DOI] [PubMed]
- 13.Lass A, Skull J, McVeigh E, et al. Measurement of ovarian volume by transvaginal sonography before ovulation induction with human menopausal gonadotrophin for in-vitro fertilization can predict poor response. Hum Reprod 1997;12:294–7. [DOI] [PubMed]
- 14.Cahill DJ, Prosser CJ, Wardle PG, et al. Relative influence of serum follicle stimulating hormone, age, and other factors on ovarian response to gonadotrophin stimulation. Br J Obstet Gynaecol 1994;101:999–1002. [DOI] [PubMed]
- 15.Mukherjee T, Copperman AB, Lapinski R, et al. An elevated day three follicle-stimulating hormone: luteinizing hormone ratio (FSH:LH) in the presence of a normal day 3 FSH predicts a poor response to controlled ovarian hyperstimulation. Fertil Steril 1996;65:588–93. [DOI] [PubMed]
- 16.American Fertility Society. Revised American Fertility Society classification of endometriosis. Fertil Steril 1985;43:351–2. [DOI] [PubMed]
- 17.Elder KT. Laboratory techniques: oocyte collection and embryo culture. In: Brinsden PR, editor. A textbook of in vitro fertilization and assisted reproduction. 2nd ed. Cambridge: Parthenon; 1999. p. 185–201.
- 18.World Health Organization. WHO laboratory manual for the examination of human semen and sperm–cervical mucus interaction. 4th ed. Cambridge, U.K: Cambridge University Press; 1999.
- 19.Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod 1997;12:1750–5. [DOI] [PubMed]
- 20.Holte J, Berglund L, Milton K, et al. Construction of an evidence based-integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod 2007;22:548–57. [DOI] [PubMed]
- 21.Steer CV. The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an in-vitro fertilization and embryo transfer programme. Hum Reprod 1992;7:117–9. [DOI] [PubMed]
- 22.The Practice Committee of the Society for Assisted Reproductive Technology and the Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril 2006;86(5 Suppl):S51–2. [DOI] [PubMed]
- 23.Baart EB, Martini E, Eijkemans MJ, et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum. Reprod 2007;22:980–8. [DOI] [PubMed]
- 24.Ludwig M, Schopper B, Katalinic A, et al. Experience with the elective transfer of two embryos under the conditions of the German embryo protection law: results of a retrospective data analysis of 2573 transfer cycles. Hum Reprod 2000;15:319–24. [DOI] [PubMed]
- 25.Gleicher N, Barad D. Twin pregnancy, contrary to consensus, is a desirable outcome in infertility. Fertil Steril. 2008;April 24 (in press). [DOI] [PubMed]
- 26.Jones HWJ, Out HJ, Hoomans EH, et al. Cryopreservation: the practicalities of evaluation. Hum Reprod 1997;12:1522–4. [DOI] [PubMed]
- 27.Polinder S, Heijnen EM, Macklon NS, et al. Cost-effectiveness of a mild compared with a standard strategy for IVF: a randomized comparison using cumulative term live birth as the primary endpoint. Hum Reprod 2008;23:316–23. [DOI] [PubMed]