Fig. 3.
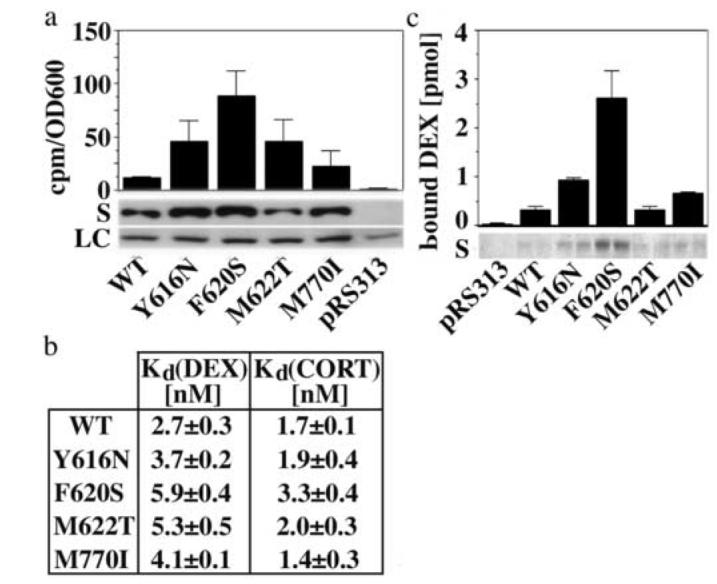
The identified mutations stabilize soluble and hormone-binding competent conformations of GR
(a) Expression of soluble GR protein (S) and in vivo accumulation of 3H-DEX (2 h incubation) in yeast (YNK410) expressing either GR WT or mutants. Shown are the averages and standard deviations of three independent experiments performed in duplicates as described in the Materials and Method section. Protein expression was monitored by immunoblot analysis using the monoclonal GR antibody BUGR2. A selected band of the Ponceau Red-stained transblot is shown as loading control (LC). (b) Dissociation constants of DEX and CORT binding reactions using GR WT and mutants expressed in reticulocyte lysate. Binding curves were measured as described in the Materials and Methods section and fit by nonlinear regression using a single site saturation binding model. Shown are the averages and standard deviations of three binding experiments per GR variant. (c) Soluble expression and 3H-DEX (15 nM) binding of recombinantly expressed and Talon purified GR WT and mutant LBDs corresponding to 100 μl bacterial culture (∼1-10 pmol). The Coomassie-stained polyacrylamide gel monitors the yield of soluble, Talon purified WT/mutant GR LBDs in two independent purifications each.
