FIGURE 8. Effect of SET knockdown on M3-MR signaling.
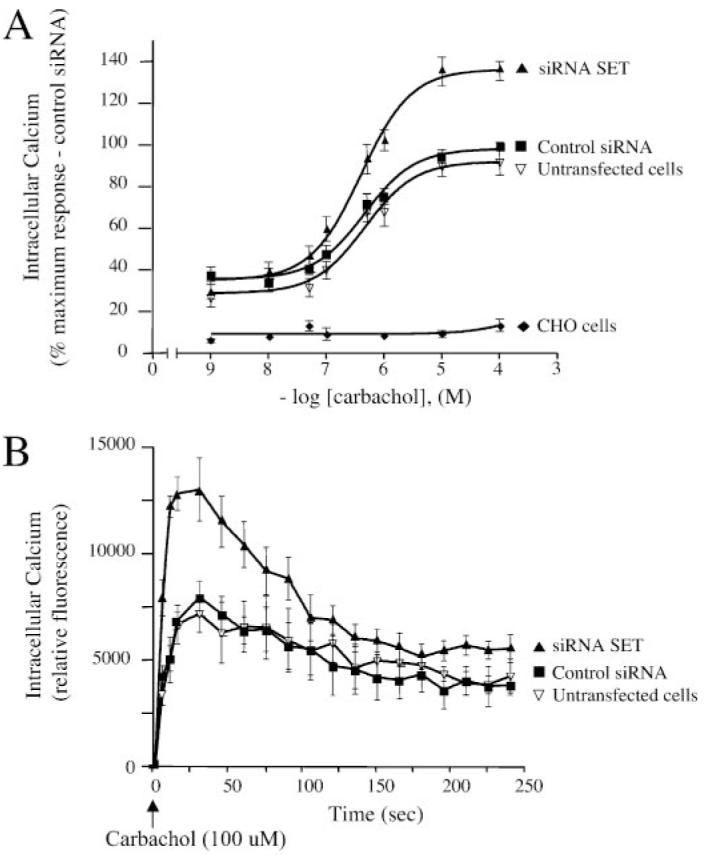
Measurement of the increases in intracellular calcium elicited by carbachol in CHO cells and CHO-M3 cells transfected or not with control siRNA oligonucleotides or SET-siRNA oligonucleotides. Cells were plated in 96-well black plates precoated with poly-D-lysine (see “Experimental Procedures”) and loaded with fluorescent dye. Excitation fluorescence was 485 nm, and emission was detected at 520 nm using a 515 nm emission cutoff filter. Six individual fields on the bottom of each well were selected for fluorescence measurement. Cells were incubated with agonist for 5 min with increasing concentrations (1 nM—100 μM) of the M3-MR agonist carbachol and fluorescence emissions were measured. A, the increases in intracellular calcium were determined by subtracting the base-line to peak values (heights). Results were expressed as the percentage of the maximal response in cells transfected with control siRNA. The data are presented as the mean ± S.E. of seven independent experiments in triplicate. Note that base-line values were not different among control cells and cells transfected with either SET siRNA or control siRNA (n = 7, mean ± S.E., untransfected cells (% control siRNA), 82.7 ± 18.8, siRNA SET (% control siRNA), 93 ± 13.6). B, time course of calcium mobilization in CHO-M3 cells after receptor activation. CHO-M3 cells were stimulated with 100 μM M3-MR agonist carbachol, and fluorescence emissions were measured for 4 min. The increases in intracellular calcium were determined by subtracting the base-line to peak values (heights). Results are expressed as relative fluorescence units. The data are expressed as the mean ± S.E. and are representative of four independent experiments in triplicate.
