Abstract
Breast cancer is immunogenic and well suited to treatment via immunomodulation. The disease is often treated to remission and time to relapse is generally measured in years in many cases. Immune-based therapeutics, such as cancer vaccines, may be able to impact the clinical progression of micrometastatic disease. Immune targets must be identified that have the potential to inhibit tumor growth. Insulin like growth factor binding protein-2 (IGFBP-2) has direct effects on breast cancer proliferation via stimulation of critical signaling pathways. We questioned whether IGFBP-2 was an immune target in breast cancer. IGFBP-2 specific IgG antibody immunity was preferentially detected in breast cancer patients as compared to controls (p=0.0008). To evaluate for the presence of T cell immunity, we identified potential pan HLADR binding epitopes derived from IGFBP-2 and tested the peptides for immunogenicity. The majority of epitopes elicited peptide specific T cells in both patients and controls and had high sequence homology to bacterial pathogens. IGFBP-2 peptide-specific T cells could respond to naturally processed and presented IGFBP-2 protein, indicating these peptides were native epitopes of IGFBP-2. Finally, both immunization with IGFBP-2 peptides as well as adoptive transfer of IGFBP-2 competent T cells mediated an anti-tumor effect in a transgenic mouse model of breast cancer. This is the first report of IGFBP-2 as a human tumor antigen and suggests the protein may serve as a relevant immunotherapeutic target in breast cancer.
Keywords: IGFBP-2, T cell, antigen, immunity, breast cancer, mimicry, therapeutics
Introduction
Breast cancer is a disease that is theoretically well suited to immunomodulation, particularly active immunization. The majority of patients respond well to standard therapies such as surgery, radiation, and chemotherapy, obtaining a complete remission or minimal residual disease state. Vaccination may be most effective with low tumor burden.(1) The time to relapse after definitive treatment in high risk patients can be measured in years rather than months, allowing the development of a potentially therapeutic immune response.(2) Moreover, breast cancer is immunogenic, and patients with the disease have been found to have pre-existent immunity to tumor-associated proteins.(3) Recent studies have suggested that immunization against one of the most well characterized breast cancer associated tumor antigens, HER-2/neu, results in resolution of HER-2/neu-overexpressing ductal carcinomas in situ in some women and may prevent the development of disease relapse in patients whose invasive breast cancers over express the protein.(4–6) Furthermore, HER-2/neu-specific antibody immunity induced by vaccinationhas been shown to inhibit protein phosphorylation and the growth of HER-2/neu overexpressing breast cancer in vitro.(7)
The success of any targeted cancer therapy depends on inhibiting the growth of cells that express the target, i.e. proteins that maintain or impact the malignant phenotype, and cancer vaccines are no exception.(8) HER-2/neu is a biologically relevant tumor antigen and aberrant signaling via the receptor is an important growth regulator for breast cancers expressing the protein. However, multiple oncogenic pathways are implicated in breast cancer progression; thus, additional immunologic targets need to be defined to enhance the therapeutic efficacy of immunization and impact tumor growth.
The insulin like growth factor (IGF) pathway is emerging as an important growth regulator in breast cancer. IGF signaling stimulates proliferation and inhibits apoptosis in cancer cells.(9) In particular, IGFBP-2 has been shown to be increasingly over expressed during breast cancer progression.(10) Recent studies have suggested that not only does IGFBP-2 have a direct proliferative effect on tumor growth, the protein is a regulator of PI3K/Akt activation and may facilitate the malignant transformation.(11–13) Stimulating immune eradication of IGFBP-2 over expressing breast cancer cells may potentially impact cancer progression.
Studies presented here demonstrate that IGFBP-2 is a human tumor antigen eliciting both antibody and T cell immunity in women with breast cancer. Moreover, in a transgenic mouse model of breast cancer, T cells specific for IGFBP-2 inhibit tumor growth. Thus, IGFBP-2 may represent a relevant target for the immunomodulation of breast cancer.
Materials and Methods
Human subjects
Serum samples were collected from 220 patients with breast cancer after written consent. Serum samples derived from 100 volunteer donors between the ages 18 and 75 years were obtained from the Puget Sound Blood Bank, Seattle, WA. The volunteers met all criteria for blood donation. All sera were aliquoted and stored at −80°C. For T cell studies, PBMCs were obtained by leukapheresis, after informed consent, from 9 breast cancer patients and one ovarian cancer patient, and 10 volunteer donors. Cells were ficolled and cryopreserved as previously described.(14)
Evaluation of humoral immunity specific for IGFBP-2
The human antibody response to IGFBP-2 was assessed by indirect ELISA as has been previously described.(15) For murine serum samples, the same protocol was used except for the following substitutions: purified mouse recombinant IGFBP-2 protein (Sigma) and anti-mouse IgG/HRP conjugate (Zymed Laboratories). IGFBP-2 antibody immunity detected by ELISA was verified using Western blot analysis.(15)
Scoring system for the prediction of MHC Class II binding epitopes
We have demonstrated that high binding affinity across multiple class II alleles predicts immunogenic human epitopes.(16) Other investigators have shown that predicted peptides that score highly across more than one algorithm are more likely to be natural epitopes.(17) Therefore, to identify IGFBP-2-specific MHC class II epitopes that have optimal binding affinity and promiscuity across multiple alleles, we developed a combined scoring system using widely available algorithms for predicting class II binding. The following five algorithms were used for prediction of Class II peptides derived from the IGFBP-2 protein sequence: SYFPEITHI (Institute for Cell Biology, Heidelberg, Germany), Propred (Institute of Microbial Technology, Chandigarh, India), MHC-Thread (University of Aberdeen, Aberdeen, UK), Average Binding matrix method, and Rankpep (Havard, Boston, MA).(18) Binding predictions were generated using each algorithm for the 15 most common MHC class II alleles: DRB1*0101, DRB1*1501, DRB1*0301, DRB1*0401, DRB1*0404, DRB1*0405, DRB1*0701, DRB1*0802, DRB1*0901, DRB1*1101, DRB1*1201, DRB1*1302, DRB3*0101, DRB4*0101, DRB5*0101.
The 14 peptides described in this study were selected as follows. For each available MHC Class II allele from the 5 algorithms, 20 peptide sequences were initially selected solely on the basis of the rank order of the predicted binding affinity. The sequences ranged from 9–15 amino acids in length. Individual amino acids for each selected peptide were assigned a score between 1 and 20, with 20 being an amino acid contained in a peptide sequence that ranked highest for predictive binding affinity across multiple algorithms. Scoring individual amino acids accounted for the multiple peptide overlaps occurring within and across algorithms. The scores (S) for each amino acid were summed up across the multiple MHC Class II alleles from all 5 algorithms. Then, the number (N) of MHC class II alleles for which each amino acid was predicted to have high affinity binding were counted. The final score for each amino acid was calculated by multiplying S and N. For ease of identifying the most potentially immunogenic segments of the IGFBP-2 protein, each amino acid was assigned a color (from dark red to light blue) based on its final score, with dark red being highest at ≥ 9,000 and light blue the lowest at 500–1,000 (Fig. 2A). Thus, the dark red color corresponds to a sequence where multiple peptides scored highly within an algorithm as well as across algorithms. Light blue represents sequences that are the least potentially immunogenic of all predicted high binding peptides. Twenty distinct 15-amino-acid peptides were chosen, representing all potential “immunogenic hot spots”. Scores (S × N) of the amino acids ranged from 0 to 9394. The sum of the scores of each selected 15-mer peptides ranged from 7,610 to 107,357. Of the 20, peptides scoring above the lower end of 99% Confidence Interval (CI) of the mean were chosen for construction and further analysis (n=14) (Fig. 2A). IGFBP-2 peptides were synthesized by Genemed Synthesis Inc. (South San Francisco, CA), purified by high-performance liquid chromatography, and characterized by mass spectrometry for use in all experiments. The 14 peptide sequences were compared in NCBI databases using BLAST program to identify shared homology with other human proteins or with proteins from other species (Table 1).
Figure 2. The majority of IGFBP-2 peptides identified by a scoring system combining multiple MHC Class II peptide binding algorithms can be recognized by human T cells.
(A) 14 peptides associated with highest binding affinity across multiple MHC class II alleles are shown. Colors represent final scores from five algorithms for each amino acid from dark red to light blue in the order of rank scores. Color strata are as follows: dark red ≥ 9,000; red = 8000–9000; orange = 7,000–8,000; light orange = 6,000–7,000; gold = 5,000–6,000; tan = 4,000–5,000; yellow = 3,000– 4,000; light yellow = 2,000–3,000; light green = 1,000–2,000; light blue = 500–1,000. (B) Percent of volunteer donors (white bars) and cancer patients (black bars) showing T cell responses to specific IGFBP-2 peptides.
Table 1.
Homology of peptides derived from IGFBP-2 protein
| IGFBP-2 peptides | % Homology with mouse IGFBP-2 | Homologous protein |
|
|---|---|---|---|
| Protein source of species | % Homology | ||
| p8-22 | 67 | A. oryzae | 67 |
| p17-31 | 0 | C. albicans | 67 |
| p67-81 | 0 | Propionibacterium acnes | 60 |
| p99-113 | 80 | Human, murine, canine IGFBP-3, IGFBP-4, IGFBP-5 | 67–80 |
| p109-123 | 80 | Pseudomonas fluorescens | 53 |
| p121-135 | 80 | P. aeruginosa | 60 |
| p164-178 | 86 | Trypanosoma cruzi | 53 |
| p190-204 | 93 | Trypanosoma cruzi | 73 |
| p213-227 | 93 | A. oryzae | 67 |
| p235-249 | 93 | C. albicans | 53 |
| p251-265 | 100 | P. aeruginosa | 47 |
| p266-280 | 100 | Lactobacillus reuteri | 47 |
| p291-305 | 93 | Schistosoma japonicum | 60 |
| p307-321 | 80 | Staphylococcus aureus | 47 |
Evaluation of T cell responses to IGFBP-2 peptides and protein and highly homologous foreign antigens
PBMCs from 20 subjects were evaluated by ELISPOT for antigen-specific IFN-γ production. Briefly, 96-well nitrocellulose plates (Millititer, Millipore, Bedford, MA) were coated overnight at 4°C with 50 µl/well of 10 µg/ml anti-human IFN-γ monoclonal antibody (clone: 1-D1K, MabTech, Nacka, Sweden) in Dulbecco's Phosphate Buffered Saline (DPBS) (Gibco Invitrogen, Carlsbad, CA). The plates were washed 3 times for 5 minutes each with 200 µl DPBS/well and blocked with 100 µl/well of 2% bovine serum albumin in DPBS for 2 hours at room temperature. PBMCs were plated at 250,000 cells per well with 10 µg/ml of the various IGFBP-2 peptides, 1 μg/ml IGFBP-2 recombinant human protein, 2.5 μg/ml of CMV or media alone in a total volume of 200 µl/well for 96 hours at 37°C in 5% CO2. The plates were washed with 200 µl of 0.05% Tween/DPBS. Wells were incubated for 2.5 hours at room temperature using 50 µl of 1 µg/ml anti-IFN-γ (clone: 7-B6-1, MabTech) antibody diluted in 0.05% Tween/DPBS. After washing three times with PBS, streptavidin-alkaline phosphatase (Bio-Rad) was diluted 1/1000 and added at 50 µl/well for 2 hours at room temperature. After another washing step with PBS, 100 μl/well of BCIP/NBT substrate (Bio-Rad) was added for up to 20 minutes. Color development was stopped by washing under running tap water. After drying overnight at room temperature, colored spots were counted using a AID ELISPOT High-Resolution reader system and AID ELISPOT Software version 3.5 (Autoimmun Diagnostika GmbH, Strasberg, Germany). The mean number of spots and SEM from six replicates at each dilution was reported for each antigen. Response to peptide antigens was considered to be positive when the mean number of spots in the experimental wells were statistically different (p<0.05) from the mean number from no antigen control wells.
For the ELISPOT assay of the cultured T cell lines, 1×105 of PBMCs, inactivated by irradiation at 3,000 rads, were added to the same number of cultured T cells per well and incubated for 24 hours at 37°C in 5% CO2. Peptide-specific T cells were assayed for IFN-γ production in the presence of IGFBP-2 peptides (25 µg/ml), IGFBP-2 protein (2.5 µg/ml), 200 μl/well of Candida albicans (Clinical grade, obtained from Hollister-Stier Lab, Spokane, WA) diluted 1:1000 in media, Pseudomonas aeruginosa, 1 μg/ml (Sigma-Aldrich, St. Louis, MO), and 1 µg/ml of PHA as positive control. HER-2/neu peptide p328–342 (25 µg/ml), myoglobin (2.5 µg/ml) and media alone served as negative controls. All assays were performed in 6 well replicates.
To assess the antigen-specific T cell responses in the vaccinated, tumor-bearing mice, 2×105 splenocytes per each well were used for the IFN-γ ELISPOT assay. Briefly, spleens were pressed through the 70-µm cell strainer (BD Labware, Franklin Lakes, NJ). The cells were washed with RPMI-1640 (Invitrogen, Grand Island, NY) and pelleted at 300g for 10 minutes. To lyse red blood cells, the pellet was resuspended in 5ml of ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.4) per spleen and incubated for 5 minutes at room temperature. Standard mouse T cell medium (described below) was added to stop the lysis buffer, and the cells were pelleted. Resultant splenocytes were stimulated by different antigens: IGFBP-2 peptides mixture (10 µg/ml of each peptides p8–31, p251–265, p291–305), murine IGFBP-2 protein (2.5 µg/ml), and 1 µg/ml of PHA as positive control. The same protocol for human IFN-γ ELISPOT was used except for the following substitutions: anti-mouse IFN-γ monoclonal antibody (clone: AN18, MabTech, Nacka, Sweden) for coating and biotinylated anti-mouse IFN-γ (clone: R4-6A2-biotin, MabTech) antibody for detection.
Generation of IGFBP-2-specific T cell lines
For the generation of human T cell lines, cryopreserved PBMC were thawed, washed, and resuspended at a concentration of 3×106 cells/ml in T cell media. The cells were stimulated with 10 µg/ml of various IGFBP-2 peptides and expanded in culture as previously described.(19, 20) For the generation of IGFBP-2-specific mouse T cell lines, pooled splenocytes from IGFBP-2 peptide vaccinated mice were used. The same protocol as for human T cell expansion was used, except that 10 ng/ml of recombinant mouse IL-7 and 5 ng/ml of recombinant human IL-15 were added on days 5 and 12. Mouse recombinant IL-7 was purchased from R&D Systems and human recombinant IL-15 from PeproTech Inc (Rocky Hill, NJ). Lyophilized recombinant IL-7 and IL-15 were reconstituted into PBS/1% BSA, aliquoted, and stored at −20°C before use. On day 26, cultured T cells were harvested and transferred to tumor bearing mice at the dose of 10×106 T cells/mouse. The same number of splenocytes derived from naïve mice were used for controls.
Phenotypic analysis of the T cells
To phenotype the IGFBP-2 peptide-specific cultured human T cells, four-color flow cytometric analysis was performed using the following antibodies (Ab): fluorescein isothiocyanate (FITC)-conjugated anti-CD8, phycoerythrin (PE)-conjugated anti-CD4, PE-Cy5-conjugated CD3, and PE-Cy7-conjugated CD56 (all from Beckman Coulter, Fullerton, CA). For extracellular staining, cells were incubated for 30 minutes at room temperature with optimal dilution of each Ab. For the analysis of FoxP3 expression in PBMCs and antigen-specific T cell lines, intracellular staining of FoxP3 using a PE-conjugated anti-human FoxP3 antibody (clone 259D, mouse IgG1, Biolegend, San Diego, CA) together with surface staining with FITC anti-CD3, PE-Cy7 anti-CD4, and PE-Cy5 anti-CD25 was performed following the manufacturer’s protocol. FACS analysis was performed using Cytomics FC 500 MPL Flow Cytometry System with CXP software (Beckman Coulter, Fullerton, CA). Typically, 50,000–100,000 events were collected per sample.
Murine experiments
Neu-transgenic mice (strain name, FVB/N-TgN (MMTVneu)-202Mul) were obtained from Charles River Laboratory (Bar Harbor, ME) and bred under SPF conditions at the University of Washington. Animal care and use were in accordance with institutional guidelines. Mice were either immunized subcutaneously with 50 µg of each IGFBP-2 peptide as a mixture in CFA/IFA (Sigma), adjuvant alone, or PBS alone. As a peptide control, in some experiments a 15-mer of a pan-HLA-DR binding peptide from tetanus toxoid was used in combination with adjuvant. Three immunizations were given two weeks apart. Two weeks after the 3rd vaccination, mice were inoculated with 1×106 mouse mammary carcinoma (MMC) cells, a cell line which had been derived from fresh spontaneous tumor from the neu-transgenic mouse, s.c. on the mid-dorsum with a 23-gauge needle.(21) To evaluate the humoral immune responses specific to murine IGFBP-2, sera from the experimental mice were taken by retro-orbital bleeding at 2 different time points: pre- and 5 weeks after tumor inoculation. In some animals, in vivo depletion of CD4+ and CD8+ T cells was performed by i.p. injection of 100 µg anti-CD8 (Clone 2.34, Santa Cruz Biotechnology, Santa Cruz, California) and 150 µg anti-CD4 (Clone GK 1.5, eBioscience) monoclonal antibodies for three consecutive days prior to the first vaccine. The treatment was repeated twice/week until termination of the study. This regimen resulted in >95% CD4+ or CD8+ T cell depletion (data not shown). For adoptive T cell experiments, tumor was established in each mouse by injecting with 1×106 MMC cells 10 days before the T cell transfer. Tumors were measured every two to three days with Vernier calipers, and tumor volume was calculated as the product of length × width × height × 0.5236. in vivo data are presented as mean ± SE of 5–10 mice/group.
Reverse transcriptase PCR analysis of IGFBP-2 mRNA expression
Total RNA from the MMC cell line was isolated using RNA4Aqueous kit (Ambion, Austin, TX). cDNA was generated from 5 µg of RNA from Superscript III reverse transcriptase (Invitrogen, San Diego, CA) with oligo-dT as primers according to manufacturer’s protocol. Five µl of 1:40 diluted cDNA was then used as a template for PCR analysis. The primer pair to amplify an 80 bp was designed based on Genebank sequences of mouse IGFBP-2, 5’-GCGCGGGTACCTGTGAAA-3’ (sense) and 5’-TCCCTCAGAGTGGTCGTCATC-3’ (antisense). The cycling conditions were as follows: 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s, 62°C for 60 s, followed by a final extension at 72°C for 4 min.
Evaluation of IGFBP-2 protein expression in murine tumors and human cell lines
Standard immunohistochemistry was performed. Briefly, 5µm thick sections of phosphate buffered saline (PBS)/formalin fixed and paraffin wax embedded murine tumor specimens were deparaffinized in xylene, rehydrated in a graded series of ethanol, and washed in distilled water. Antigen retrieval was achieved by placing the tissues in 0.01M citrate buffer at pH 6.0 and exposing them to repeated microwave heating for periods of 10 minutes at high power. The specimens were cooled at room temperature for 15 minutes and washed in PBS (pH 7.6) three times. Endogenous peroxidase or phosphatase activity was quenched by incubation in 3% H2O2/PBS for 30 minutes, followed by blocking of non-specific antibody binding in 10% goat serum for 30 minutes at room temperature. Tissue sections were then incubated overnight at 4°C in a humidifier with primary goat anti-IGFBP2 (Santa Cruz Biotechnology, polyclonal antibody diluted 1/200 in 1% bovine serum albumin). 1% bovine serum albumin in PBS was used to incubate negative controls. After washing with PBS, sections were incubated with a biotinylated anti-goat secondary antibody (Vector Labs, Burlingame, CA) at room temperature for 1 hour. Avidin-conjugated peroxidase (DAKO) was added at room temperature for 30 minutes after washing with PBS. Finally, slides washed with PBS, and developed with DAB (DAKO). After terminating the reaction, sections were counterstained with freshly filtered hematoxylin and mounted.
MCF-7 and SKBR3 human breast cancer cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The MCF-7 cells were cultured in Eagle's Minimum Essential Medium (GIBCO, Grand Island, NY) supplemented by 10% fetal bovine serum and antibiotics (100 units/ml penicillin and 100 µg/ml streptomycin) at 37°C in a humidified 5% CO2 atmosphere. The SKBR3 cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum and antibiotics. Cells were harvested at confluency, pelleted, and stored at −80°C until use. Anti-human IGFBP-2 antibody and anti-β-actin antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) for Western blot. Cell lysates were made as previously described for MCF-7, SKBR3, MMC, and syngeneic splenocytes.(22) Commercially provided T98G cell lysate (Santa Cruz Biotechnology) was used as a positive control. Lysates, 10 μg/each, were resolved on 12% SDS-PAGE gel, and transferred onto nitrocellulose membranes (Amersham Pharmacia Biotech., Little Chalfont, UK). The membranes were blocked with 5% non-fat milk/TBS for 1 hour, and incubated overnight with each primary antibody (1:500) at 4°C. After washing, the membranes were incubated for 1 hour with a peroxidase-labeled secondary antibody (Amersham Pharmacia Biotech., 1:5,000) at room temperature. After rewashing, the bands were visualized using a peroxidase-linked enhanced chemiluminescence detection system (Amersham Pharmacia Biotech.).
Statistical analysis
The unpaired, two-tailed Student’s t-test was used to evaluate differences in T cell responses in ELISPOT assay, antibody responses between patients and volunteer donors, and differences in tumor growth between animal treatment groups. The relationship between the degree of homology and the immunogenicity of the peptides was analyzed by the Spearman correlation analysis. The Chi-square trend and the Student’s t-test were used to compare the magnitude and pattern of T cell responses in cancer patients and volunteer donors. In all cases, a p value less than 0.05 was considered significant. All statistical analyses were performed using GraphPad Prism version 3.02 (GraphPad Software, San Diego, CA).
Results
Breast cancer patients can have antibody immunity to IGFBP-2
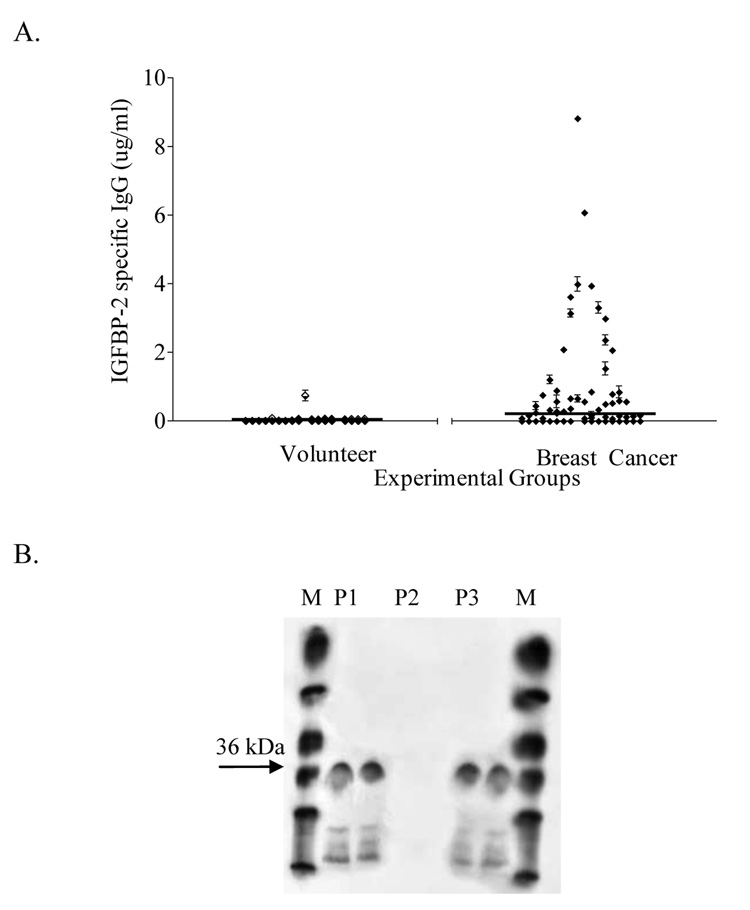
Sera from patients with breast cancer were more likely to have antibody immunity specific for IGFBP-2 than samples from volunteer donors (p=0.0008) (Fig. 1A). Moreover, the level of antibody response to IGFBP-2 was significantly higher in breast cancer patients (p=0.0008) when compared to volunteer donors. The mean level of IGFBP-2-specific IgG immunity for breast cancer patients was 0.3 µg/ml (range, 0–10.4), and for volunteer donors was 0.032 µg/ml (range, 0–1.6 µg/ml). Antibody responses were confirmed by Western blot. A representative example of two patient samples positive for IGFBP-2 antibodies (P1, P3) in ELISA and one negative patient sample (P2) are shown in duplicates (Fig. 1B).
Figure 1. Breast cancer patients can have antibody immunity to IGFBP-2.
(A) IGFBP-2 ELISA results for 220 breast cancer patients and 100 normal donors; mean and standard error of 3 replicates. Lines: mean level of IGFBP-2-specific IgG immunity for each sample group. (B) Western blot analysis of representative samples positive and negative by indirect ELISA. M: molecular weight marker; P1, P3: replicates of ELISA-positive patient samples; P2: replicates of ELISA-negative sample; Arrow: IGFBP-2 36 kD.
The majority of IGFBP-2 peptides identified by a scoring system combining multiple MHC Class II peptide binding algorithms can be recognized by human T cells
Figure 2A depicts the entire IGFBP-2 protein sequence and the identified immunogenic “hot spots”. Ten (71%) of the 14 evaluated peptides stimulated significant IFN-γ ELISPOT responses in volunteer donors and cancer patients (Fig. 2B). Of the 14 peptides, 36% elicited responses in cancer patients and 50% were immunogenic in volunteer donors. Fourteen percent of the peptides elicited responses in both cancer patients and volunteer donors. Due to the high incidence of T cell responses to IGFBP-2 peptides in volunteer donors, we evaluated the peptides for sequence homology with other known proteins. The majority (13/14) of peptides displayed significant sequence homology with bacterial pathogens. Eight of the 14 immunogenic IGFBP-2 peptides demonstrated ≥ 60% shared homology with common pathogens, such as Pseudomonas and Aspergillus (Table 1). Moreover, several peptides had multiple homologies to infectious proteins (e.g. p8–22, see below). However, there was no relationship between the degree of homology and the immunogenicity of the peptides (Spearman r=0.088, p=0.76).
Human T-cell responses specific for IGFBP-2 peptides can be restricted to a single peptide or demonstrate multiple specificities. Eight of 20 subjects (40%) showed IGFBP-2 peptide specific IFN-γ producing T-cell responses to one or more peptides. Representative examples are shown in Figure 3. Twelve out of 20 (60%) had no detectable immunity to any IGFBP-2 peptide (e.g. Fig. 3A). Half of the responding donors (n=4) demonstrated immune responses restrictive to single epitope (e.g. Fig. 3B) and the other half had polyclonal responses to multiple epitopes (e.g. Fig. 3C). There were no significant differences between cancer patients and volunteer donors in terms of pattern of response (χ2=3.125, p=0.077) or magnitude (p=0.48) of the IGFBP-2 peptide specific T cell response.
Figure 3. Human T-cell responses specific for IGFBP-2 peptides can be restricted to a single peptide or demonstrate multiple specificities.
Three patterns of T cell response to IGFBP-2 epitopes were identified in tested subjects: (A) no response, (B) dominant response to one epitope, and (C) responses to multiple epitopes. Antigens tested include IGFBP-2 peptides (grey bars), CMV positive control (black bars), and media only (white bars). Data are expressed as the mean and standard deviation of IFN-γ-secreting spots for six replicates; * denotes p < 0.05 versus spots obtained from media only wells.
IGFBP-2 peptide specific T cells respond to IGFBP-2 and highly homologous proteins
We questioned whether IGFBP-2 peptide specific T cells could respond to IGFBP-2 protein and, thus, represent native epitopes. PBMCs from one breast cancer patient and two volunteer donors who demonstrated peptide-specific T cell responses were selected for T cell expansion. Each subject had a response to a different peptide: p8–22, p251–265, or p291–305. The IGFBP-2 p8–22 T cell line responded significantly to IGFBP-2 protein as compared to human myoglobin, a control protein (p=0.0022) (Fig. 4A). In addition to Aspergillus, the IGFBP-2 peptide p8–22 has a 60% homology to candida albicans and 47% homology to pseudomonas aeruginosa and also demonstrated specificity to both those proteins (p=0.026 and 0.039 respectively) (Fig. 4B). Similarly, IGFBP-2 p251–265 (Fig. 4C) and p291–305 (Fig. 4D) T cell lines responded to the recombinant protein as compared to control (p=0.002 and p=0.0022 respectively). All of the peptide-specific T cell lines showed specific reactivity by IFN-γ secretion in response to their stimulating IGFBP-2 peptide and not to HER-2/neu p328–342, a control 15-mer peptide (p <0.05).
Figure 4. IGFBP-2 peptide-specific T cells respond to IGFBP-2 protein.
Antigen-specific responses of IGFBP-2 peptide-specific T cell lines (p8–22, A; p251–265, C; and p291–305, D) were analyzed by IFN-γ ELISPOT. Antigens tested include IGFBP-2 peptides and protein (dark gray bars), PHA positive control (black bar), HER2 p384–398 peptide and myoglobin as negative controls (light gray bars), and media alone (white bar). (B) the p8–22 response (gray bar) against candida and pseudomonas (black bars) with media alone (white bar). Data are expressed as the mean and standard deviation of IFN-γ-secreting spots for six replicates; * denotes p < 0.05 versus spots obtained from negative control wells.
The IGFBP-2-specific T cell lines were predominantly composed of CD4+ T cells (mean 53.6%, range 46.4–66.3%). CD8+ T cells (mean 36.0%, range 25.1–43.7%) and cells double negative for CD4+ and CD8+ (mean 6.7%, range 4.0–9.5%) accounted for the rest of the cell population. None of the cultured T cell lines demonstrated outgrowth of regulatory T cells. The mean percentage of T regulatory cells was 0.7% (range 0.2–1.1%) after in vitro expansion.
IGFBP-2 specific immunity inhibits tumor growth in neu-transgenic mice
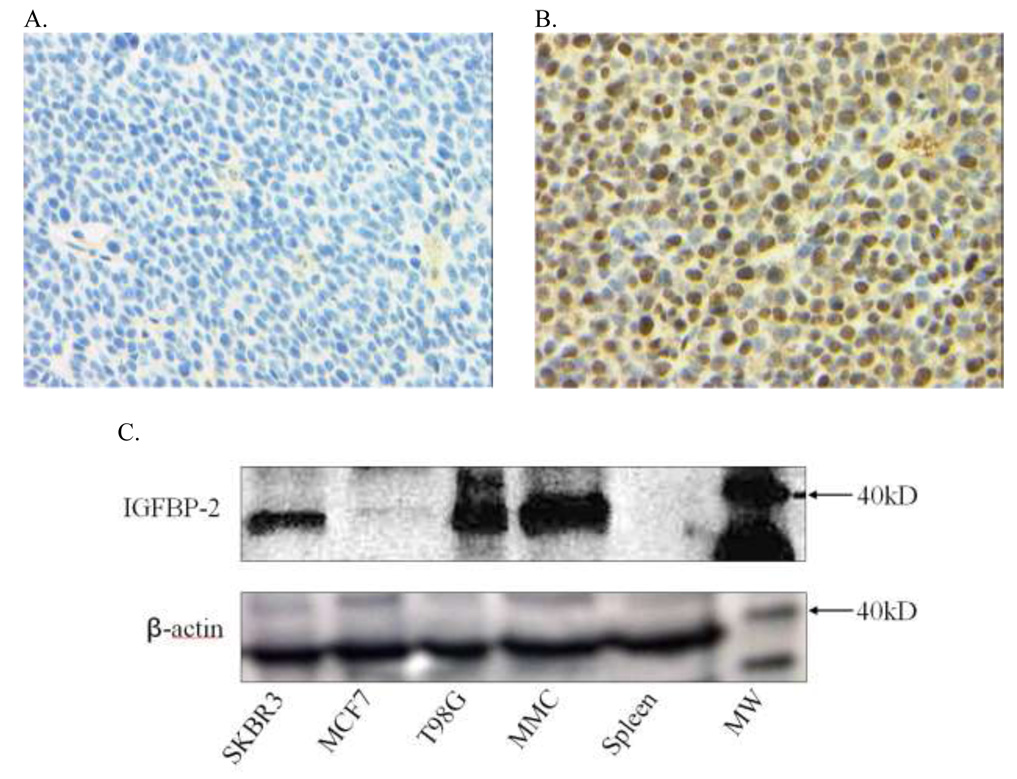
Human IGFBP-2 has a high degree of homology with murine IGFBP-2 (82%) and for this reason we questioned whether immunity to IGFBP-2 would impact tumor growth. IGFBP-2 peptides p8–22, p251–265, and p291–305 were chosen for in vivo study because they were shown to be native epitopes of human IGFBP-2, and all have significant homology with murine IGFBP-2 protein (Table 1). IGFBP-2 mRNA is expressed in both the MMC cell line and fresh spontaneous tumor from neu-transgenic mice (data not shown) so we evaluated protein expression in the tumors. Immunohistochemical staining demonstrated diffuse intracellular IGFBP-2 staining in MMC (Fig. 5B) as compared to isotype control (Fig. 5A). Western blot analysis was performed to compare the amount of IGFBP-2 protein in the MMC tumor to two commonly studied human breast cancer cell lines, MCF-7 and SKBR3 (Fig. 5C). IGFBP-2 protein was expressed in human breast cancer cell lines at approximately the same level as in MMC, but could not be detected in murine splenocytes (Fig. 5C).
Figure 5. IGFBP-2 protein expression in tumor cells derived from neu-transgenic mice.
IGFBP-2 monoclonal antibody staining of MMC cells (A) isotype control, (B) IGFBP-2 protein expression, magnification 400X. (C) Top panel is western blot of 10 µg SKBR3, MCF-7, T98G, MMC, and neu-transgenic mouse spleen lysate. Arrow: 40 kD level of molecular weight marker (IGFBP-2 36 kD). Bottom panel represents beta-actin expression in 10 µg of the same lysates.
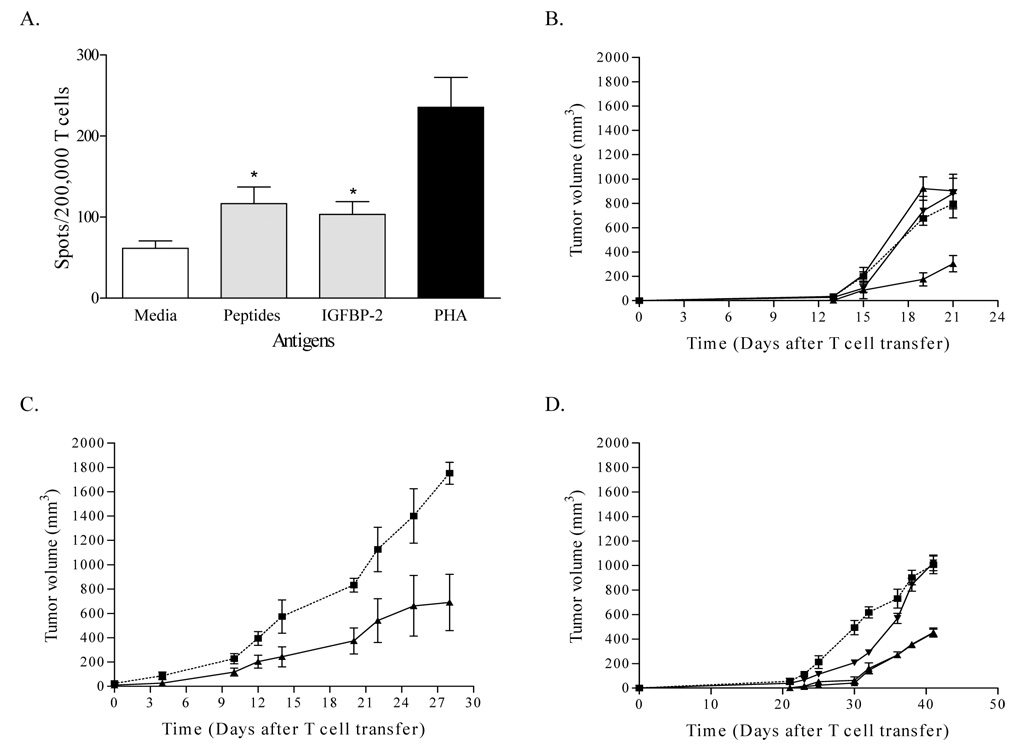
Animals were immunized with a vaccine composed of all three peptides. The peptides were immunogenic in the mice. The vaccine generated both peptide and murine IGFBP-2 protein-specific IFN-γ secreting T cells (Fig. 6A). IFN-γ ELISPOT responses were significantly higher to the peptide mix (p=0.0022) and murine recombinant protein (p=0.002) as compared to no antigen wells.
Figure 6. IGFBP-2 specific immunity inhibits tumor growth in neu-transgenic mice.
(A) T cell responses specific for the vaccinated IGFBP-2 peptides and IGFBP-2 protein were identified in IFN-γ ELISPOT using splenocytes from vaccinated mice. Columns represent the mean spots for six replicates. Bars indicate SD; * denotes p < 0.05 versus spots obtained from media-only wells. (B) Shown are tumor measurements from mice injected with IGFBP-2 peptide vaccines (•), tetanus toxoid peptide (▼), adjuvant alone (▲), or PBS (■). Each data point represents the mean tumor measurement ± SD from 10 mice. (C) Shown is tumor growth for mice treated with IGFBP-2 peptide-specific T cells (▲) or naïve splenocytes (■) 10 days following tumor challenge. Each data point represents the mean tumor measurement ± SD from 5 mice. (D) Tumor growth in mice injected with (■) PBS alone (◆) IGFBP-2 vaccine and CD8+ depletion, (▼) IGFBP-2 vaccine and CD4+ depletion, and (▲) IGFBP-2 vaccination with no depletion. Each data point represents the mean tumor measurement ± SD from 5 mice.
IGFBP-2 peptide vaccination inhibited tumor growth by approximately 50% compared with control groups (Fig. 6B). The differences of mean tumor size between the IGFBP-2 peptide vaccinated group on day 21 (mean±SD; 304±66-mm3), tetanus toxoid peptide vaccinated group (mean±SD; 881±126-mm3), adjuvant alone (mean±SD; 902±136-mm3) and PBS control group (mean±SD; 796±115-mm3) were statistically significant (PBS vs. IGFBP-2 vaccine, p=0.035; adjuvant alone vs. IGFBP-2 vaccine, p=0.035; tt peptide vs. IGFBP-2 vaccine, p=0.035). To assess the therapeutic efficacy of IGFBP-2-specific T cells, 1×107 of in vitro cultured IGFBP-2 peptide specific T cells were adoptively transferred to 10-day tumor bearing mice. A single infusion of IGFBP-2 specific T cells inhibited tumor growth by 60% (Fig. 6C). Twenty eight days after T cell transfer, the mean tumor size of the IGFBP-2 specific T cell treated group (mean±SD; 691±231-mm3) was significantly different from that of animals receiving an equal dose infusion of naïve splenocytes (mean±SD; 1751±90-mm3, p=0.015).
To further determine which T cell subset was the mediator of the anti-tumor effect, mice were selectively depleted of CD4+ and CD8+ T cells just prior to and during IGFBP-2 immunization. As shown in Figure 6D, the anti-tumor effect of vaccination was impacted by CD8+ T cell depletion but not by CD4+ T cell depletion. The average tumor size in mice depleted of CD8+ T cells was 1062±90-mm3, significantly larger than the tumors in mice depleted of CD4+ T cells (454±82-mm3, p=0.02) or non-depleted animals (427±35-mm3, p=0.02).
Discussion
Insulin like growth factor binding proteins, such as IGFBP-2, transport IGFs from the circulation into tissues and are part of an important regulatory network controlling cell proliferation, migration, and apoptosis.(23) IGFBP-2 is one of six IGFBPs and is found at elevated levels in the sera of cancer patients.(24–26) Studies have demonstrated that IGFBP-2 has an IGF independent growth stimulatory effect on tumor cells, directly promoting cell growth while inhibiting apoptosis.(27) More recently IGFBP-2 has been shown to act as a regulatory protein for PTEN in breast cancer cells.(13) Elevated levels of IGFBP-2 prevent PTEN interaction with IGFR-II, thus resulting in enhanced cell proliferation via activation of the PI3K/Akt signaling pathway. In breast cancer, IGFBP-2 expression has not been found in normal glandular tissue but has been found in increasing levels in pre-malignant and malignant disease with the highest levels associated with invasive ductal carcinomas.(10) We questioned whether IGFBP-2 might be a target for immunomodulation in breast cancer and whether the ability to recognize IGFBP-2 was within the realm of the human T cell repertoire. Data presented here demonstrate that not only is IGFBP-2 a human tumor antigen but also that an IGFBP-2-specific T cell response may inhibit tumor growth in vivo.
Initially we evaluated whether patients with breast cancer developed IGFBP-2 IgG antibody immunity which we theorized would be a marker for a potential cellular immune response as immunoglobulin class switching from IgM to IgG requires cognate CD4+ T-cell help.(28, 29) Moreover, it has been shown that tumor antigen-specific antibody immunity is positively associated with a concomitant antigen-specific T-cell response, indicating that IgG immunity may act as a marker for the presence of CD4+ and CD8+ T-cell immunity.(30, 31) Previous work by our group demonstrated that those peptides most likely to be native epitopes of the tumor antigen HER-2/neu bound at high affinity across multiple class II alleles.(16) For this reason, we analyzed the IGFBP-2 protein sequence using 5 class II prediction algorithms across multiple class II alleles and developed a scoring system that would maximize the identification of peptides with predicted promiscuous high affinity class II binding. The majority of peptides identified in this fashion elicited a T cell response, indicating this approach may be a useful tool in class II epitope prediction.
The identified IGFBP-2 class II peptides demonstrated a high degree of homology with common bacterial pathogens. As a comparison, HER-2/neu peptides shown to elicit T cell responses in vitro and in vivo did not demonstrate such structural similarity to bacterial antigens (data not shown), nor have these HER-2/neu peptides been shown to be immunogenic in non-tumor bearing individuals.(32) Structural similarities between sequences derived from microorganisms and self epitopes has been termed “molecular mimicry,” which is a suggested explanation for some autoimmune diseases.(33) Indeed, T cell specificities that are cross reactive with both self and bacterial antigens have been implicated in the pathogenesis of diabetes and multiple sclerosis.(34, 35) Molecular mimicry of peptide sequences derived from foreign organisms with self tumor antigens has been identified as one potential reason for the immunogenicity of melanoma antigens such as MART-1.(36) A dominant HLA-A2 class I epitope derived from MART-1 and capable of eliciting cytotoxic T cells with the ability to lyse tumor was highly homologous and cross reactive with an HSV-1 peptide. It is unknown what role molecular mimicry plays in the immunogenicity of IGFBP-2. Potentially the bacterial and self sequences may share HLA-Class II binding anchor residues. The high incidence of detectable immunity in non-tumor bearing individuals, however, would suggest that the T cell responses observed may not be due entirely to autoimmunization via exposure to an IGFBP-2 expressing malignancy.
IGFBP-2 is immunogenic in patients with breast cancer, but does that immune response have an impact on tumor growth? We asked this question using the neu-transgenic mouse model. Neu-transgenic mice are engineered to express non-transforming rat neu on an MMTV promoter.(37) The breast cancer that occurs in these mice is histologically similar to breast cancer in humans. Hyperplastic lesions progress to infiltrating ductal carcinomas, which commonly metastasize to local lymph nodes and soft tissue sites. Moreover, the tumors that develop are estrogen receptor low and demonstrate tamoxifen resistance.(38) IGFBP-2 is expressed in these tumors. Both active immunization with IGFBP-2 peptides as well as adoptive transfer of IGFBP-2 competent T cells mediated an anti-tumor response in treated mice as compared to controls. Although results in mice cannot be directly comparable to humans, the model has many immunologic similarities with human breast cancer. T regulatory cells are operative in dampening immunity to the neu antigen; therefore, immunization against IGFBP-2 circumvented tolerance.(39) T regulatory cells have been shown to play an important role in the progression of human breast cancer.(40) Inflammatory infiltrates develop as the tumor progresses in neu transgenic mice just as such infiltrates develop in human breast cancers.(41, 42) Finally, the antigenic repertoire in the neu-transgenic mouse appears to be quite similar to that found in patients with malignancy.(42) Thus, studies presented here may lay the foundation for the development of a vaccine targeting IGFBP-2 in patients with breast cancer.
Of note, although mice were immunized with putative class II epitopes, the effector cell mediating the anti-tumor response was the CD8+ T cell. It has long been known that T helper (Th) cells activate antigen-specific effector cells and recruit cells of the innate immune system such as macrophages, eosinophils, and mast cells which can enhance cross priming.(43, 44) Moreover, antigen primed Th cells can directly activate tumor antigen-specific cytotoxic T cells (CTL). Investigations have demonstrated that the infusion of Th cell clones into tumor-bearing animals can activate a CTL-mediated anti-tumor response.(45) Other studies have demonstrated that the function of tumor-specific CTL is enhanced by Th cells through co-stimulatory molecules present on the surface of the CTL such as CD27, CD134, and MHC.(46) In addition to direct contact, Th can activate CTL through cytokine secretion which can stimulate the growth and expansion of effector T cells. For example, Th1 cells release IFN-γ which activates antigen presenting cells to upregulate molecules such as LMP2, LMP7, MECL, PA28, and MHC class I, all of which contribute to increased antigen presentation to CTL.(47) In addition, Th1 induce the production of opsonizing antibodies that enhance the uptake of tumor cells into antigen presenting cells promoting expansion of tumor-specific CTL. Data presented here demonstrates immunization with class II epitopes may be an efficient method for delivering antigen specific T help required to initiate and sustain therapeutic CTL responses.
Despite an increasing identification of human tumor antigens there is still little insight as to which targets may potentially elicit an anti-tumor response. Indeed, evidence in murine models suggests that some tumor-associated proteins may actually serve to inhibit immunity by inducing the elaboration of T regulatory cells in an attempt to prevent an autoimmune response.(48) There is a need for the identification of biologically relevant immunogenic proteins that may ultimately serve as tumor rejection antigens. IGFBP-2 has a direct growth stimulating effect on breast cancer cells, is expressed in a majority of breast cancers, is immunogenic in breast cancer patients, and immunity against the protein can significantly inhibit tumor growth in a biologically relevant animal model. Therefore, IGFBP-2 may be an important target for the immune modulation of breast cancer.
Acknowledgments
This work was supported by a grant from the Ovarian Cancer Research Fund and a gift from Athena Water as well as NIH grants U54 CA090818 and R01 CA101190. Subject specimens were collected at the General Clinical Research Center Facility at the University of Washington (NIH MO1-RR-00037). We thank Ms. Sally Zebrick and Molly Boettcher for assistance in manuscript preparation.
Abbreviations
- Ab
antibody
- CMV
cytomegalovirus
- CTL
cytotoxic T lymphocyte
- ELISPOT
enzyme-linked immunospot
- HER2
HER-2/neu
- IFA
Incomplete Freund’s Adjuvant
- IGFBP
insulin-like growth factor binding protein
- IGF
insulin like growth factor
- IGFR
insulin-like growth factor receptor
- MMC
mouse mammary carcinoma
- NCBI
National Center for Biotechnology Information
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- Th
T helper
References
- 1.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 2.Day RS, Shackney SE, Peters WP. The analysis of relapse-free survival curves: implications for evaluating intensive systemic adjuvant treatment regimens for breast cancer. Br J Cancer. 2005;92:47–54. doi: 10.1038/sj.bjc.6602267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disis ML, Knutson KL, Schiffman K, Rinn K, McNeel DG. Pre-existent immunity to the HER-2/neu oncogenic protein in patients with HER-2/neu overexpressing breast and ovarian cancer. Breast Cancer Res Treat. 2000;62:245–252. doi: 10.1023/a:1006438507898. [DOI] [PubMed] [Google Scholar]
- 4.Peoples GE, Gurney JM, Hueman MT, et al. Clinical trial results of a HER2/neu (E75)vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23:7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 5.Czerniecki BJ, Koski GK, Koldovsky U, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67:1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 6.Salazar LG, Knutson KL, Dang Y, Dela Rosa C, Disis ML. Long-term immunity after immunization with a HER-2/neu vaccine; AACR Meeting Proc; 2004. (Abstr.) [Google Scholar]
- 7.Montgomery RB, Makary E, Schiffman K, Goodell V, Disis ML. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005;65:650–656. [PubMed] [Google Scholar]
- 8.Kamb A, Wee S, Lengauer C. Why is cancer drug discovery so difficult? Nat Rev Drug Discov. 2007;6:115–120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- 9.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 10.Busund LT, Richardsen E, Busund R, et al. Significant expression of IGFBP2 in breast cancer compared with benign lesions. J Clin Pathol. 2005;58:361–366. doi: 10.1136/jcp.2004.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehrian-Shai R, Chen CD, Shi T, et al. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci U S A. 2007;104:5563–5568. doi: 10.1073/pnas.0609139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin JL, Baxter RC. Expression of Insulin-Like Growth Factor Binding Protein-2 byMCF-7 Breast Cancer Cells Is Regulated through the Phosphatidylinositol 3-Kinase/AKT/Mammalian Target of Rapamycin Pathway. Endocrinology. 2007;148:2532–2541. doi: 10.1210/en.2006-1335. [DOI] [PubMed] [Google Scholar]
- 13.Perks CM, Vernon EG, Rosendahl AH, Tonge D, Holly JM. IGF-II and IGFBP-2differentially regulate PTEN in human breast cancer cells. Oncogene. 2007 doi: 10.1038/sj.onc.1210397. [DOI] [PubMed] [Google Scholar]
- 14.Disis ML, dela Rosa C, Goodell V, et al. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods. 2006;308:13–18. doi: 10.1016/j.jim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Goodell VMD, Disis ML. His-tag ELISA for the detection of humoral tumor-specific immunity. BMC Immunology. 2008;9:23. doi: 10.1186/1471-2172-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salazar LG, Fikes J, Southwood S, et al. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res. 2003;9:5559–5565. [PubMed] [Google Scholar]
- 17.Lu J, Celis E. Use of two predictive algorithms of the world wide web for the identification of tumor-reactive T-cell epitopes. Cancer Res. 2000;60:5223–5227. [PubMed] [Google Scholar]
- 18.Bui HH, Sidney J, Peters B, et al. Automated generation and evaluation of specific MHC binding predictive tools: ARB matrix applications. Immunogenetics. 2005;57:304–314. doi: 10.1007/s00251-005-0798-y. [DOI] [PubMed] [Google Scholar]
- 19.Dang Y, Knutson KL, Goodell V, et al. Tumor antigen-specific T-cell expansion is greatly facilitated by in vivo priming. Clin Cancer Res. 2007;13:1883–1891. doi: 10.1158/1078-0432.CCR-06-2083. [DOI] [PubMed] [Google Scholar]
- 20.Knutson KL, Disis ML. IL-12 enhances the generation of tumour antigen-specific Th1 CD4 T cells during ex vivo expansion. Clin Exp Immunol. 2004;135:322–329. doi: 10.1111/j.1365-2249.2004.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutson KL, Lu H, Stone B, et al. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–1533. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 22.Goodell V, Disis ML. Human tumor cell lysates as a protein source for the detection of cancer antigen-specific humoral immunity. J Immunol Methods. 2005;299:129–138. doi: 10.1016/j.jim.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 24.Baron-Hay S, Boyle F, Ferrier A, Scott C. Elevated serum insulin-like growth factor binding protein-2 as a prognostic marker in patients with ovarian cancer. Clin Cancer Res. 2004;10:1796–1806. doi: 10.1158/1078-0432.ccr-0672-2. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Nicar MR, Shi R, et al. Levels of insulin-like growth factor I (IGF-I) and IGF binding proteins 2 and 3 in serial postoperative serum samples and risk of prostate cancer recurrence. Urology. 2001;57:471–475. doi: 10.1016/s0090-4295(00)01003-7. [DOI] [PubMed] [Google Scholar]
- 26.Renehan AG, Jones J, Potten CS, Shalet SM, O'Dwyer ST. Elevated serum insulin-like growth factor (IGF)-II and IGF binding protein-2 in patients with colorectal cancer. Br J Cancer. 2000;83:1344–1350. doi: 10.1054/bjoc.2000.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schutt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol. 2004;32:859–868. doi: 10.1677/jme.0.0320859. [DOI] [PubMed] [Google Scholar]
- 28.Nakatsura T, Senju S, Ito M, Nishimura Y, Itoh K. Cellular and humoral immune responses to a human pancreatic cancer antigen, coactosin-like protein, originally defined by the SEREX method. Eur J Immunol. 2002;32:826–836. doi: 10.1002/1521-4141(200203)32:3<826::AID-IMMU826>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Di Modugno F, Bronzi G, Scanlan MJ, et al. Human Mena protein, a serex-defined antigen overexpressed in breast cancer eliciting both humoral and CD8+ T-cell immune response. Int J Cancer. 2004;109:909–918. doi: 10.1002/ijc.20094. [DOI] [PubMed] [Google Scholar]
- 30.Gnjatic S, Atanackovic D, Jager E, et al. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci U S A. 2003;100:8862–8867. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jager D, Taverna C, Zippelius A, Knuth A. Identification of tumor antigens as potential target antigens for immunotherapy by serological expression cloning. Cancer Immunol Immunother. 2004;53:144–147. doi: 10.1007/s00262-003-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Disis ML, Gooley TA, Rinn K, et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 33.Blank M, Barzilai O, Shoenfeld Y. Molecular mimicry and auto-immunity. Clin Rev Allergy Immunol. 2007;32:111–118. doi: 10.1007/BF02686087. [DOI] [PubMed] [Google Scholar]
- 34.Uemura Y, Senju S, Maenaka K, et al. Systematic analysis of the combinatorial nature of epitopes recognized by TCR leads to identification of mimicry epitopes for glutamic acid decarboxylase 65-specific TCRs. J Immunol. 2003;170:947–960. doi: 10.4049/jimmunol.170.2.947. [DOI] [PubMed] [Google Scholar]
- 35.Croxford JL, Olson JK, Anger HA, Miller SD. Initiation and exacerbation of autoimmune demyelination of the central nervous system via virus-induced molecular mimicry: implications for the pathogenesis of multiple sclerosis. J Virol. 2005;79:8581–8590. doi: 10.1128/JVI.79.13.8581-8590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loftus DJ, Castelli C, Clay TM, et al. Identification of epitope mimics recognized by CTL reactive to the melanoma/melanocyte-derived peptide MART-1(27-35) J Exp Med. 1996;184:647–657. doi: 10.1084/jem.184.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menard S, Aiello P, Tagliabue E, et al. Tamoxifen chemoprevention of a hormone-independent tumor in the proto-neu transgenic mice model. Cancer Res. 2000;60:273–275. [PubMed] [Google Scholar]
- 39.Knutson KL, Dang Y, Lu H. IL-2 immunotoxin therapy modulates tumor-associated regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in neu-transgenic mice. J Immunol. 2006;177:84–91. doi: 10.4049/jimmunol.177.1.84. [DOI] [PubMed] [Google Scholar]
- 40.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 41.Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol. 2006;59:972–977. doi: 10.1136/jcp.2005.031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu H, Knutson KL, Gad E, Disis ML. The tumor antigen repertoire identified in tumor-bearing Neu transgenic mice predicts human tumor antigens. Cancer Res. 2006;66:9754–9761. doi: 10.1158/0008-5472.CAN-06-1083. [DOI] [PubMed] [Google Scholar]
- 43.Cohen PA, Peng L, Plautz GE, Kim JA, Weng DE, Shu S. CD4+ T cells in adoptive immunotherapy and the indirect mechanism of tumor rejection. Crit Rev Immunol. 2000;20:17–56. [PubMed] [Google Scholar]
- 44.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J Immunol. 2000;164:562–565. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fallarino F, Grohmann U, Bianchi R, Vacca C, Fioretti MC, Puccetti P. Th1 and Th2 cell clones to a poorly immunogenic tumor antigen initiate CD8+ T cell-dependent tumor eradication in vivo. J Immunol. 2000;165:5495–5501. doi: 10.4049/jimmunol.165.10.5495. [DOI] [PubMed] [Google Scholar]
- 46.Giuntoli RL, 2nd, Lu J, Kobayashi H, Kennedy R, Celis E. Direct costimulation of tumor-reactive CTL by helper T cells potentiate their proliferation, survival, and effector function. Clin Cancer Res. 2002;8:922–931. [PubMed] [Google Scholar]
- 47.Fruh K, Yang Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr Opin Immunol. 1999;11:76–81. doi: 10.1016/s0952-7915(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 48.Nishikawa H, Kato T, Tanida K, et al. CD4+ CD25+ T cells responding to serologically defined autoantigens suppress antitumor immune responses. Proc Natl Acad Sci USA. 2003;100:10902–10906. doi: 10.1073/pnas.1834479100. [DOI] [PMC free article] [PubMed] [Google Scholar]