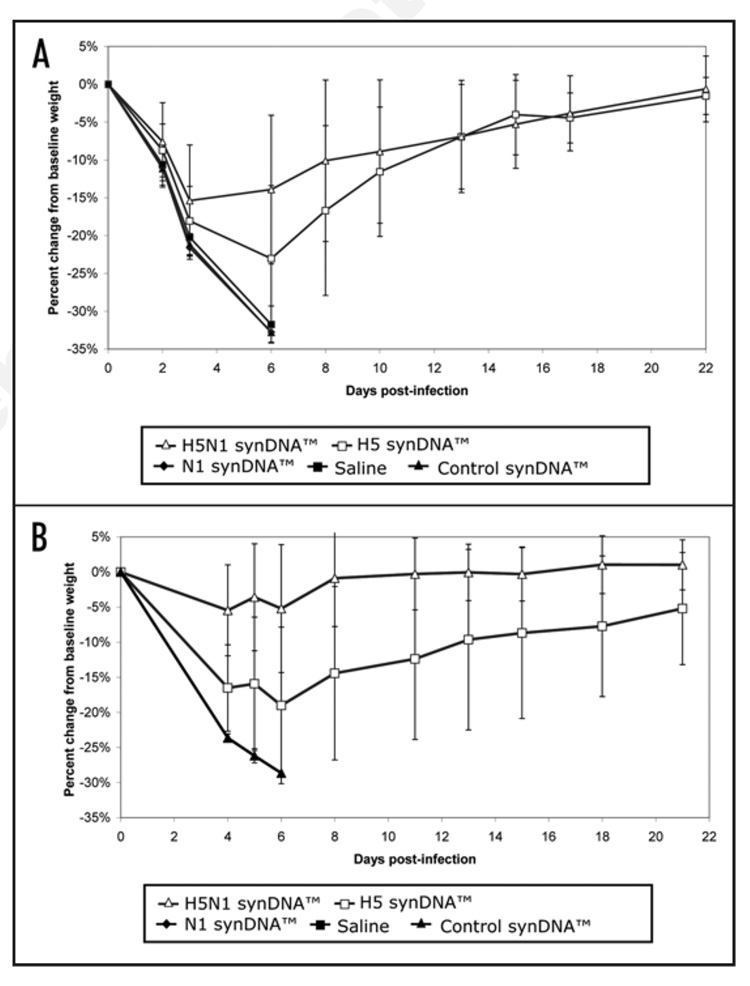
Figure 4.
Percent change in body weight of synDNA™ vaccinated mice following challenge with influenza A/H5N1. In two independent trials of synDNA™ vaccine efficacy in mice (survival and details presented in Fig. 3), periodic recording of body weight was performed over the 21–22 day study period. Briefly, mice were vaccinated via intramuscular (i.m.) route with the indicated synDNA™ vaccine (50 µg per mouse) or, as controls, with saline or control synDNA™, on day -42, -28 and -14 relative to H5N1 challenge. Two weeks post-immunization (day 0), mice were challenged with a lethal dose of influenza A/H5N1(Vietnam/1203/04). Figure 4A and B represent the results of two independent vaccine efficacy trials, as described in Figure 3A and B, respectively. The percent change in body weight relative to baseline (day 0) was calculated for individual mice and the percent change averaged by group. Each data point represents the average of 15 mice as indicated for H5N1 synDNA™, H5 synDNA™, N1 synDNA™, control synDNA™ and saline groups.