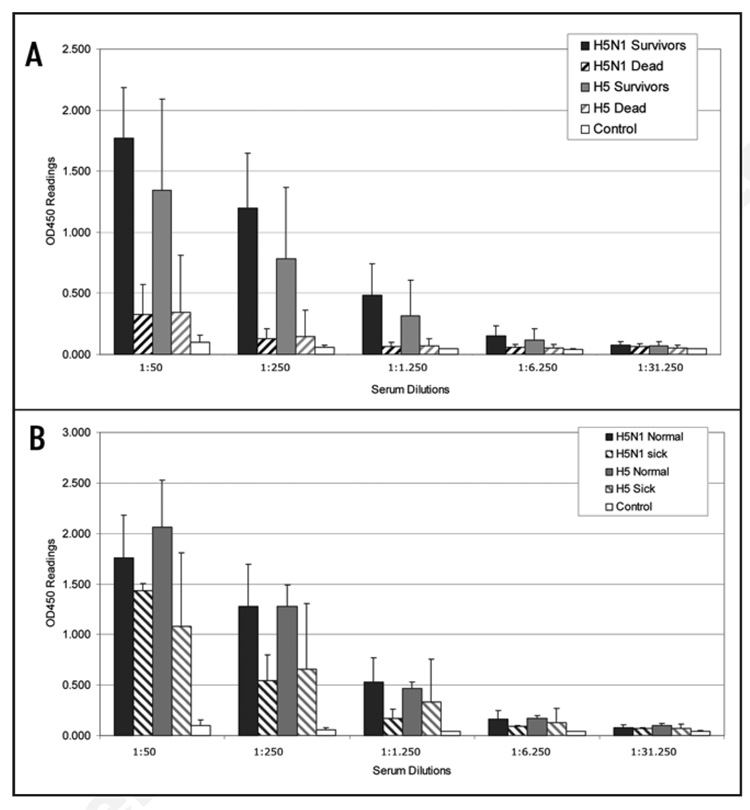
Figure 8.
Serological analysis of synDNA™ vaccinated mice prior to H5N1 challenge (summary from trials 1 and 2). Mice were vaccinated via intramuscular (i.m.) route with the indicated synDNA™ (50 µg per mouse) or, as controls, with saline or control synDNA™, on day -42, -28 and -14 relative to H5N1 challenge. Serum from H5N1, H5 and control syn-DNA™ immunized mice was collected prior to challenge (on day 0, two weeks following third vaccination dose) and analyzed for the presence of anti-H5 antibodies by ELISA. Error bars represent standard deviations. (A) Anti-H5 antibody titers in mice that survived the lethal challenge with H5N1 virus are represented by solid bars for H5N1 synDNA™ (black; n = 27, see Table 1) and H5 synDNA™ (grey; n = 21, see Table 1). Hatched bars depict antibody titers in H5N1 and H5 synDNA™ immunized mice that did not survive the challenge (black, n = 3 and grey, n = 9, respectively. See Table 1). Anti-H5 antibody titers in mice immunized with control synDNA™ are represented by white solid bars (n = 7, for reagent sparing purposes anti-H5 antibody titers were assessed in only 6 animals from the control group in trial 1 and 1 in trial 2). (B) Comparative analysis of anti-H5 antibody titers in the pre-challenge sera of mice that survived the challenge without detectable infection symptoms (normal) and in morbid mice that recovered by the end of the trial (sick). N = 22, H5N1 normal; n = 5, H5N1 sick; n = 10, H5 normal; n = 11, H5 sick; n = 7, control (see Table 1).