Figure 4. Perifosine induces PKCα and c-Jun activation in THP-1 cells.
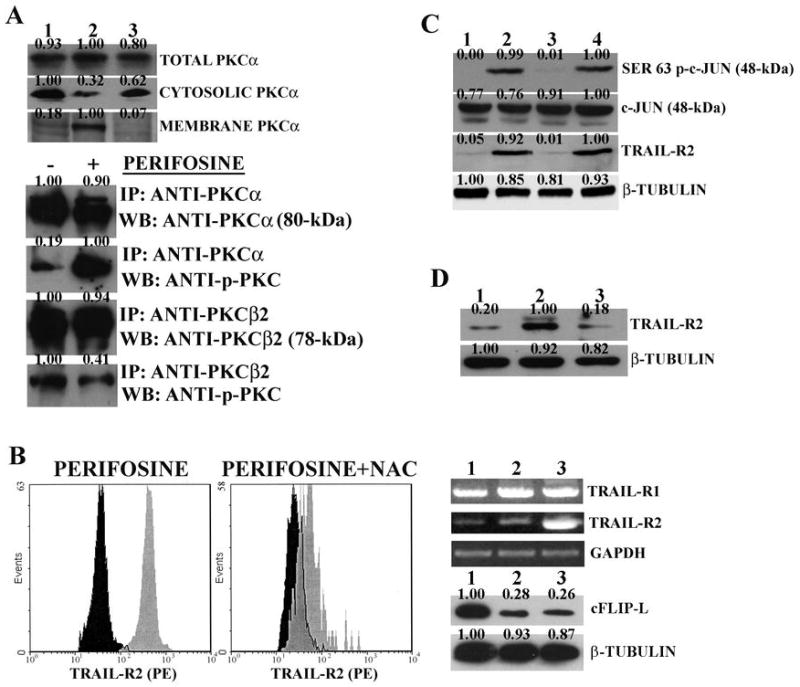
A: western blot analysis for PKCα expression in different subcellular fractions. Lane 1: untreated cells; Lane 2: cells treated with perifosine (2 μM for 16 hr); Lane 3: cells treated with perifosine (2 μM for 16 hr) + NAC (15 mM). Immunoprecipitation of cell extracts from untreated and perifosinetreated (2 μM for 16 hr) samples. IP: antibody used for immunoprecipitation; WB: antibody used for probing the blots. B: flow cytometric analysis RT-PCR, and western blot. Flow cytometric analysis shows surface expression of TRAIL-R2 receptor in untreated cells (black shaded histograms) and cells treated with 2.0 μM perifosine for 16 h (grey shaded histograms) with or without NAC (15 mM). In case of RT-PCR analysis for TRAIL-R1 and –R2 mRNA, perifosine and NAC concentrations were as for flow cytometry. Lane 1: untreated cells; Lane 2: NAC +perifosine; Lane 3: perifosine alone. In case of western blot analysis for cFLIP-L expression, lanes were as for RT-PCR analysis. C: western blot analysis for Ser 63 p-c-Jun, c-Jun, and TRAIL-R2 levels in cells treated with perifosine (2 μM for 16 hr) in the presence or in the absence of JNK 1/2 or p38 MAP kinase inhibitors. Lane 1: untreated cells. Lane 2: perifosine only; Lane 3: perifosine + SP600125 (10 μM); Lane 4: perifosine + SB203580 (5 μM). D: western blot analysis for TRAIL-R2 in cells with downregulated c-Jun levels. Lane 1: untreated cells; Lane 2: cells treated with perifosine (2 μM for 16 hr); Lane 3: perifosine-treated cells with c-Jun downregulated by siRNA specific for c- Jun. β-tubulin served as loading control.
