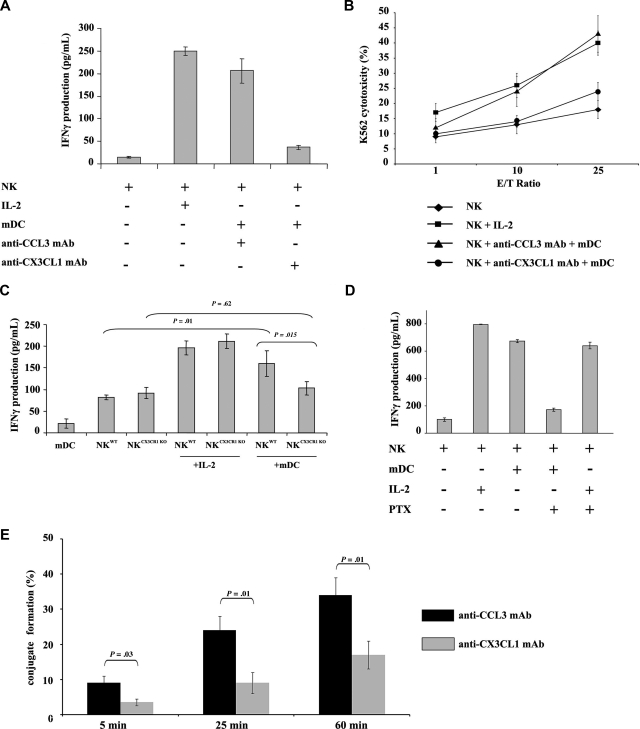
Figure 1.
CX3CL1 is required for NK activation by mDCs. (A) Autologous resting human NK cells (105) and mDCs (104) were cultured in 96-well plates at 37°C in a humidified 5% CO2 incubator in AIMV medium for 24 hours. CX3CL1 or CCL3-blocking mAb were added to the cultures. NK-derived IFN-γ production was assessed in culture supernatants by ELISA. (B) After NK and mDC cocultures, NK cells were counted and cytotoxic functions were assessed against K562 target cells. Data represent means of triplicates plus or minus the standard error (SE). Five independent experiments were performed with similar results. (C) To confirm a role for CX3CR1 signaling in DC-mediated NK cell activation, murine CX3CR1-deficient and wild-type NK cells were cultured with syngeneic wild-type bone marrow–derived DCs for 24 hours. IFN-γ production was determined. Results of a representative experiment out of 3 are expressed as the mean of triplicate assays with error bars representing standard deviations from the mean. (D) Human PTx-treated NK cells were cultured with mDCs or IL-2 for 24 hours. Supernatants were harvested to assess IFN-γ production. The depicted data represent means of triplicates plus or minus SE of a representative experiment out of 5 performed. (E) The ability of mDCs to form tight conjugates with resting NK cells was studied in the presence or absence of anti-CX3CL1 mAb. mDCs were admixed with resting NK cells (at a 1:2 mDC/NK ratio) and analyzed by transmission light microscopy as described in “Methods.” More than 100 DCs per experiment were examined for their capacity to bind to resting NK cells. The percentages of DCs forming conjugates with NK cells are shown as a mean plus or minus SE of 3 independent experiments.