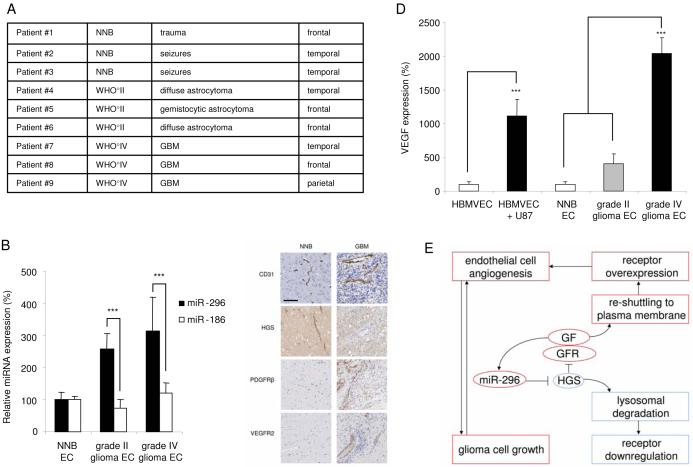
Fig. 8.
Analysis of expression of miR-296 and its targets in tumor endothelial cells isolated from human gliomas (A) RNA was isolated from primary endothelial cell cultures (passage 0) prepared from normal human brain (NNB; N = 3), grade II astrocytomas (N = 3) and grade IV glioblastoma multiforme (N = 3). (B) RNA extracted from individual tumor endothelial samples was analyzed by qRT-PCR for expression levels of miR-296 and miR-186. All values were normalized to GAPDH mRNA levels in the same samples. (C) Immunohistochemical staining of brain blood vessels indicate increased and morphologically abnormal tumor blood vessels, as well as differential HGS, PDGFR-β, and VEGFR2 expression in non-neoplastic brain (NNB) and malignant glioma (GBM). Size bar 100 μm. (D) RNA extracted from individual tumor endothelial samples was analyzed by qRTPCR for expression levels of VEGF. All values were normalized to GAPDH mRNA levels in the same samples. (E) Schematic overview of the proposed angiogenic mechanism of miR-296. miR-296 is up-regulated in glioma endothelial cells and directly inhibits expression of HGS, thereby allowing the accumulation of growth factor receptors by attenuating their degradation. GF, growth factor; GFR, growth factor receptor. Error bars indicate S.D., ***p < 0.001, t test.