Figure 4.
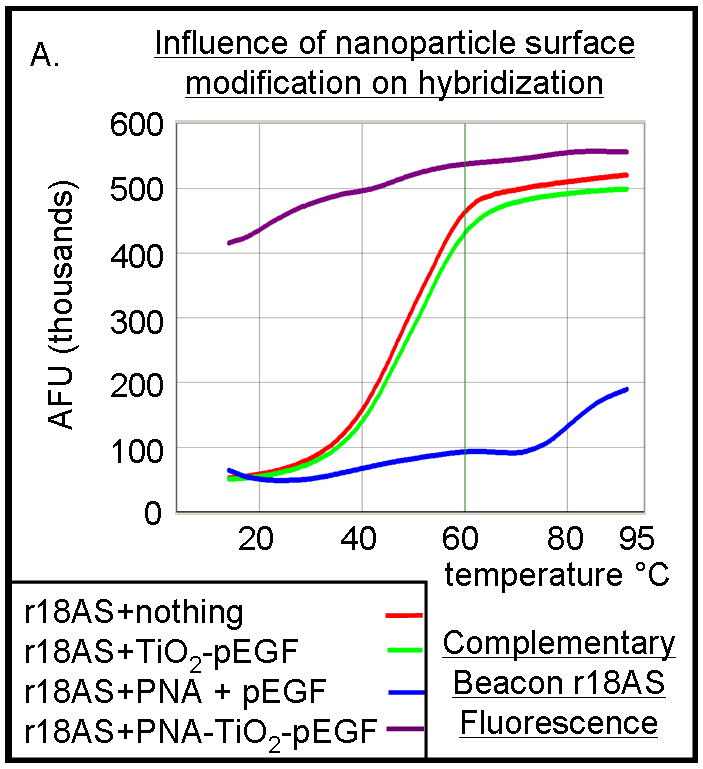
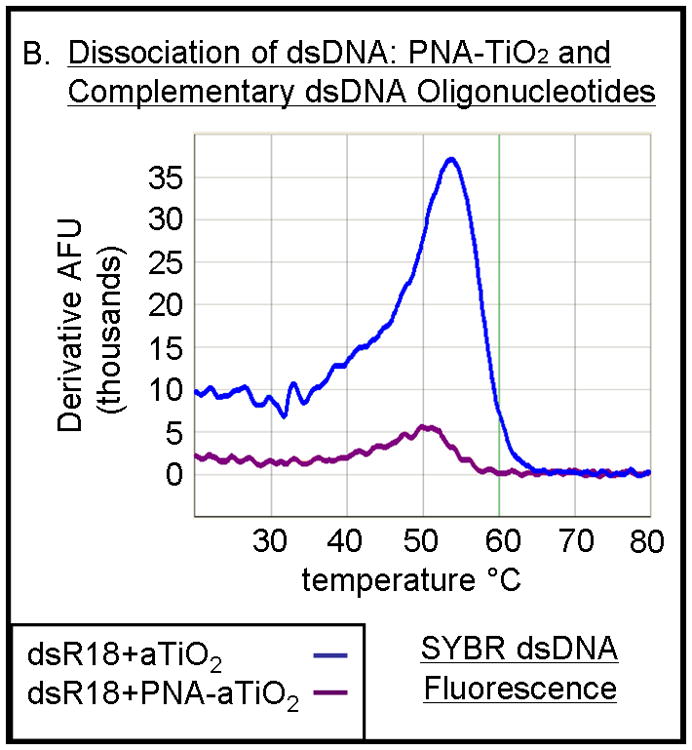
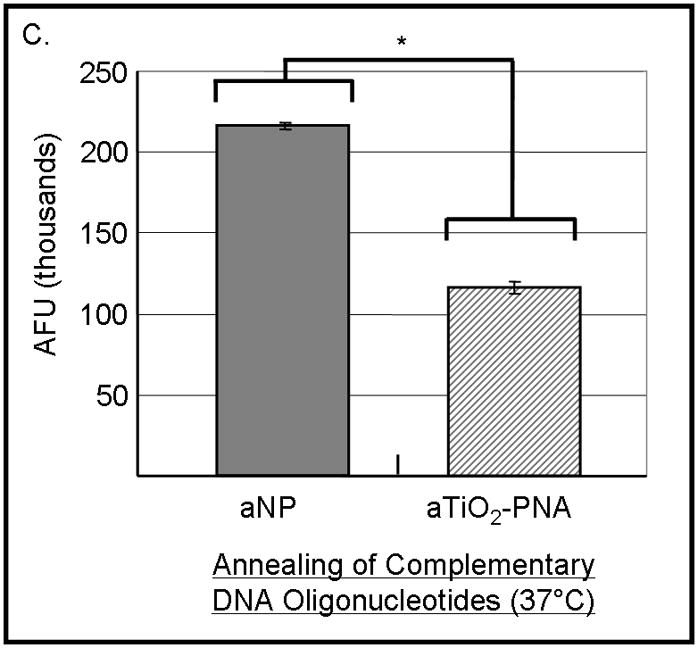
PNA-TiO2 nanoconjugates (PNA-TiO2) retain their hybridization abilities after additional modification of the nanoparticle. (A) PNA-TiO2 nanoconjugates conjugated with a peptide that represents a segment of epidermal growth factor (pEGF) maintain the ability to hybridize to target molecular beacon. Representative dissociation curves resulting from hybridization-dissociation reactions containing various test samples and a complementary molecular beacon. Hybridization of either PNA-TiO2 or PNA to molecular beacon r18AS results in alterations in fluorescence curves. (B) Alizarin red s-coated PNA-TiO2 nanoconjugates (PNA-aTiO2) maintain the ability to outcompete homologous DNA oligonucleotide from a dsR18 dsDNA oligonucleotide. Derivative dissociation curves show a lowering of Tm peak height associated with addition of PNA-aTiO2 to complementary DNA hybridization mixture. (C) Alizarin red s-coated PNA-TiO2 nanoconjugates (PNA-aTiO2) are able to engage in strand exchange with dsDNA at 37° C under 137mM sodium conditions. AFU = arbitrary fluorescent units; TiO2 = nanoparticle; PNA = PNA; pEGF = a peptide that represents a segment of epidermal growth factor; PNA-TiO2-pEGF = PNA-TiO2 nanoconjugates additionally coated with a peptide that represents a segment of epidermal growth factor; dsR18 = r18S/r18AS dsDNA hybrid; aTiO2 = Alizarin red s coated nanoparticle; PNA-aTiO2 = PNA and Alizarin red s nanoconjugates. * = p <0.05
