Abstract
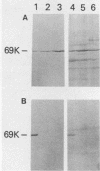
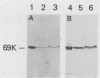
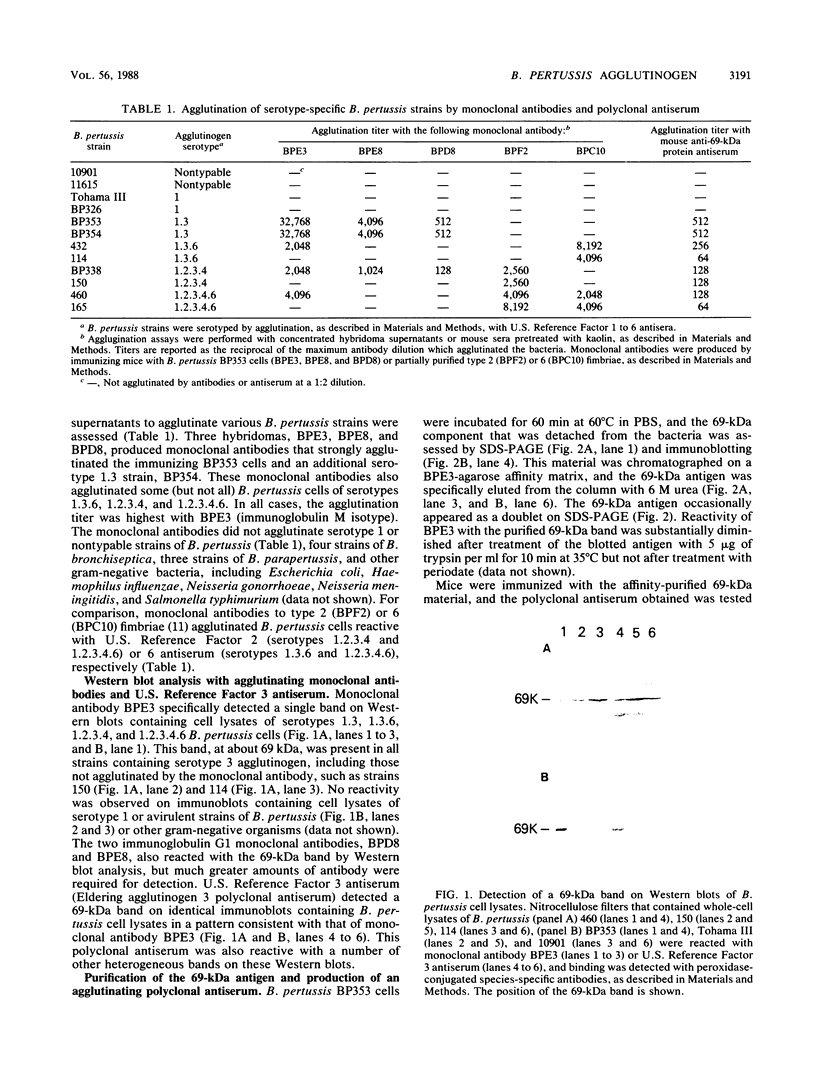
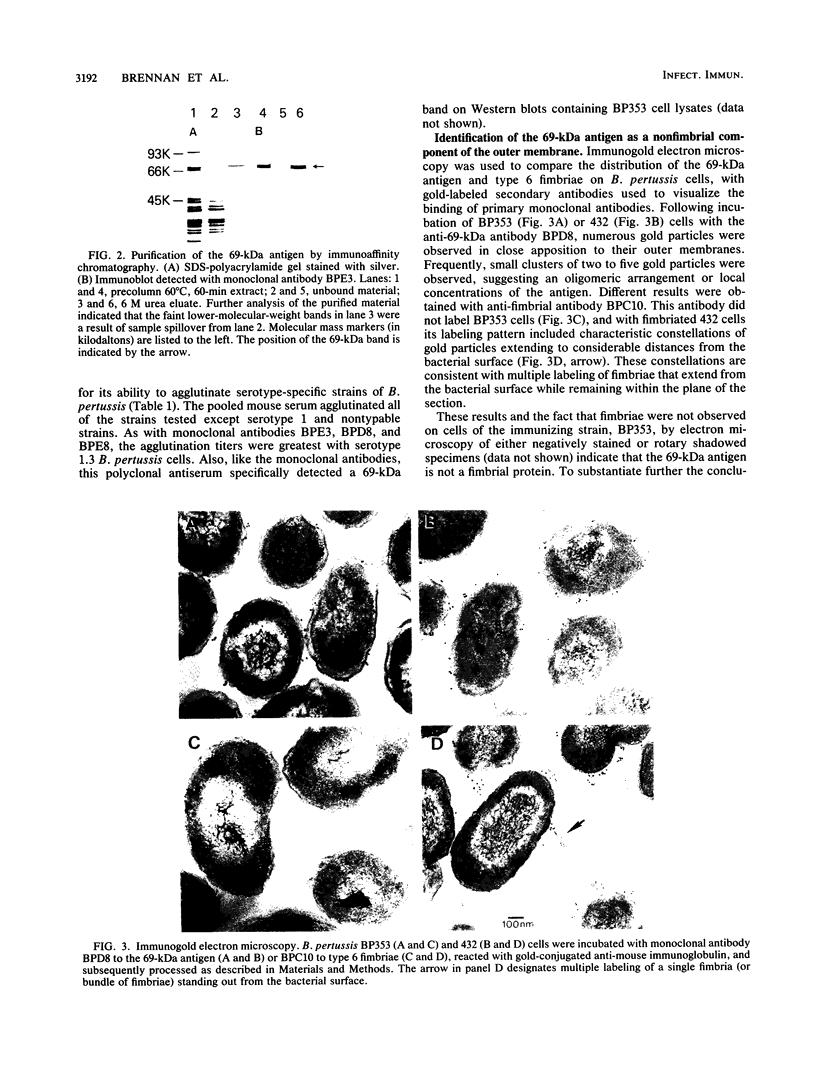
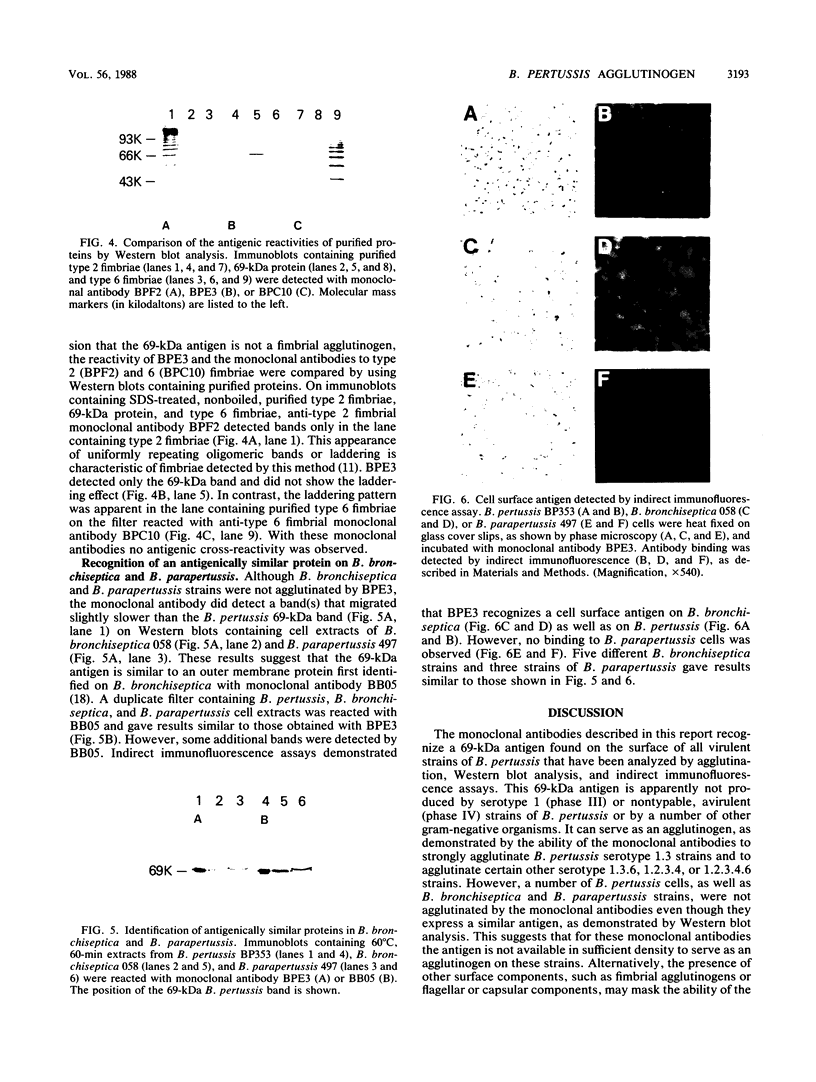
Cells of Bordetella pertussis BP353, a nonfimbriated Eldering serotype 1.3 strain, were used as an immunogen to produce three monoclonal antibodies, BPE3, BPD8, and BPE8, that agglutinated the immunizing cells, as well as certain other nonfimbriated and fimbriated serotype 3-containing B. pertussis strains. The antibodies did not agglutinate serotype 1 or nontypable B. pertussis cells. These monoclonal antibodies specifically detected a 69-kilodalton (kDa) band on Western blots (immunoblots) containing whole B. pertussis cell lysates of Eldering agglutinogen serotypes 1.3, 1.3.6, 1.2.3.4, and 1.2.3.4.6. This 69-kDa antigen was released from the bacteria by cell incubation for 60 min at 60 degrees C, and it was purified by affinity chromatography with a BPE3-agarose affinity matrix. Purified material was used to produce a polyclonal antiserum that agglutinated all nonfimbriated and fimbriated B. pertussis cells containing serotype 3 agglutinogen. Immunogold electron microscopy and indirect immunofluorescence studies demonstrated that it is an outer membrane constituent but nonfimbrial in appearance. BPE3 did not detect purified fimbriae on Western blots, and antibodies to these fimbriae did not bind to the 69-kDa component. Although B. bronchiseptica and B. parapertussis cells were not agglutinated by the monoclonal antibodies, antigenically similar proteins were detected in extracts of the bacteria. These results identify the 69-kDa protein as a nonfimbrial agglutinogen present on all virulent strains of B. pertussis. The monoclonal antibodies described here should be useful for further studies on the structure and function of this protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN E. K. Serological studies on H. pertussis H. parapertussis and H. bronchisepticus. Acta Pathol Microbiol Scand. 1953;33(2):202–224. doi: 10.1111/j.1699-0463.1953.tb01512.x. [DOI] [PubMed] [Google Scholar]
- Armstrong S. K., Parker C. D. Heat-modifiable envelope proteins of Bordetella pertussis. Infect Immun. 1986 Oct;54(1):109–117. doi: 10.1128/iai.54.1.109-117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth L. A., Irons L. I., Dowsett A. B. Antigenic relationship between serotype-specific agglutinogen and fimbriae of Bordetella pertussis. Infect Immun. 1982 Sep;37(3):1278–1281. doi: 10.1128/iai.37.3.1278-1281.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell J. L., Zhang J. M., Urisu A., Suzuki A., Steven A. C., Liu T., Liu T. Y., Manclark C. R. Purification and characterization of serotype 6 fimbriae from Bordetella pertussis and comparison of their properties with serotype 2 fimbriae. Infect Immun. 1987 Apr;55(4):916–922. doi: 10.1128/iai.55.4.916-922.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDERING G., HORNBECK C., BAKER J. Serological study of Bordetella pertussis and related species. J Bacteriol. 1957 Aug;74(2):133–136. doi: 10.1128/jb.74.2.133-136.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldering G., Holwerda J., Davis A., Baker J. Bordetella pertussis serotypes in the United States. Appl Microbiol. 1969 Oct;18(4):618–621. doi: 10.1128/am.18.4.618-621.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P. E., Clarkson J. A. Reflections on the efficacy of pertussis vaccines. Rev Infect Dis. 1987 Sep-Oct;9(5):866–883. doi: 10.1093/clinids/9.5.866. [DOI] [PubMed] [Google Scholar]
- Irons L. I., Ashworth L. A., Robinson A. Release and purification of fimbriae from Bordetella pertussis. Dev Biol Stand. 1985;61:153–163. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Z. M., Brennan M. J., David J. L., Carter P. H., Cowell J. L., Manclark C. R. Comparison of type 2 and type 6 fimbriae of Bordetella pertussis by using agglutinating monoclonal antibodies. Infect Immun. 1988 Dec;56(12):3184–3188. doi: 10.1128/iai.56.12.3184-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. M., Cowell J. L., Brennan M. J., Burns D. L., Manclark C. R. Agglutinating monoclonal antibodies that specifically recognize lipooligosaccharide A of Bordetella pertussis. Infect Immun. 1988 Mar;56(3):699–702. doi: 10.1128/iai.56.3.699-702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaraz J. A., Novotny P., Ivanyi J. Identification of a 68-kilodalton protective protein antigen from Bordetella bronchiseptica. Infect Immun. 1985 Mar;47(3):744–751. doi: 10.1128/iai.47.3.744-751.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., van der Heide H. G., ter Avest A. R., Welinder K. G., Livey I., van der Zeijst B. A., Gaastra W. Characterization of fimbrial subunits from Bordetella species. Microb Pathog. 1987 Jun;2(6):473–484. doi: 10.1016/0882-4010(87)90054-4. [DOI] [PubMed] [Google Scholar]
- Novotny P., Chubb A. P., Cownley K., Montaraz J. A. Adenylate cyclase activity of a 68,000-molecular-weight protein isolated from the outer membrane of Bordetella bronchiseptica. Infect Immun. 1985 Oct;50(1):199–206. doi: 10.1128/iai.50.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny P., Chubb A. P., Cownley K., Montaraz J. A., Beesley J. E. Bordetella adenylate cyclase: a genus specific protective antigen and virulence factor. Dev Biol Stand. 1985;61:27–41. [PubMed] [Google Scholar]
- Novotny P., Kobisch M., Cownley K., Chubb A. P., Montaraz J. A. Evaluation of Bordetella bronchiseptica vaccines in specific-pathogen-free piglets with bacterial cell surface antigens in enzyme-linked immunosorbent assay. Infect Immun. 1985 Oct;50(1):190–198. doi: 10.1128/iai.50.1.190-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston N. W. Potency tests for pertussis vaccines: doubtful value of intracerebral challenge test in mice. J Pathol Bacteriol. 1966 Jan;91(1):173–179. doi: 10.1002/path.1700910121. [DOI] [PubMed] [Google Scholar]
- Preston N. W., Surapatana N., Carter E. J. A reappraisal of serotype factors 4, 5 and 6 of Bordetella pertussis. J Hyg (Lond) 1982 Feb;88(1):39–46. doi: 10.1017/s0022172400069874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge T. N., Preston N. W. Variation of serotype in strains of Bordetella pertussis. J Hyg (Lond) 1974 Oct;73(2):305–310. doi: 10.1017/s0022172400024165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Bisher M. E., Trus B. L., Thomas D., Zhang J. M., Cowell J. L. Helical structure of Bordetella pertussis fimbriae. J Bacteriol. 1986 Sep;167(3):968–974. doi: 10.1128/jb.167.3.968-974.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Genetic studies of the molecular basis of whooping cough. Dev Biol Stand. 1985;61:11–19. [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zhang J. M., Cowell J. L., Steven A. C., Carter P. H., McGrath P. P., Manclark C. R. Purification and characterization of fimbriae isolated from Bordetella pertussis. Infect Immun. 1985 May;48(2):422–427. doi: 10.1128/iai.48.2.422-427.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. M., Cowell J. L., Steven A. C., Manclark C. R. Purification of serotype 2 fimbriae of Bordetella pertussis and their identification as a mouse protective antigen. Dev Biol Stand. 1985;61:173–185. [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]