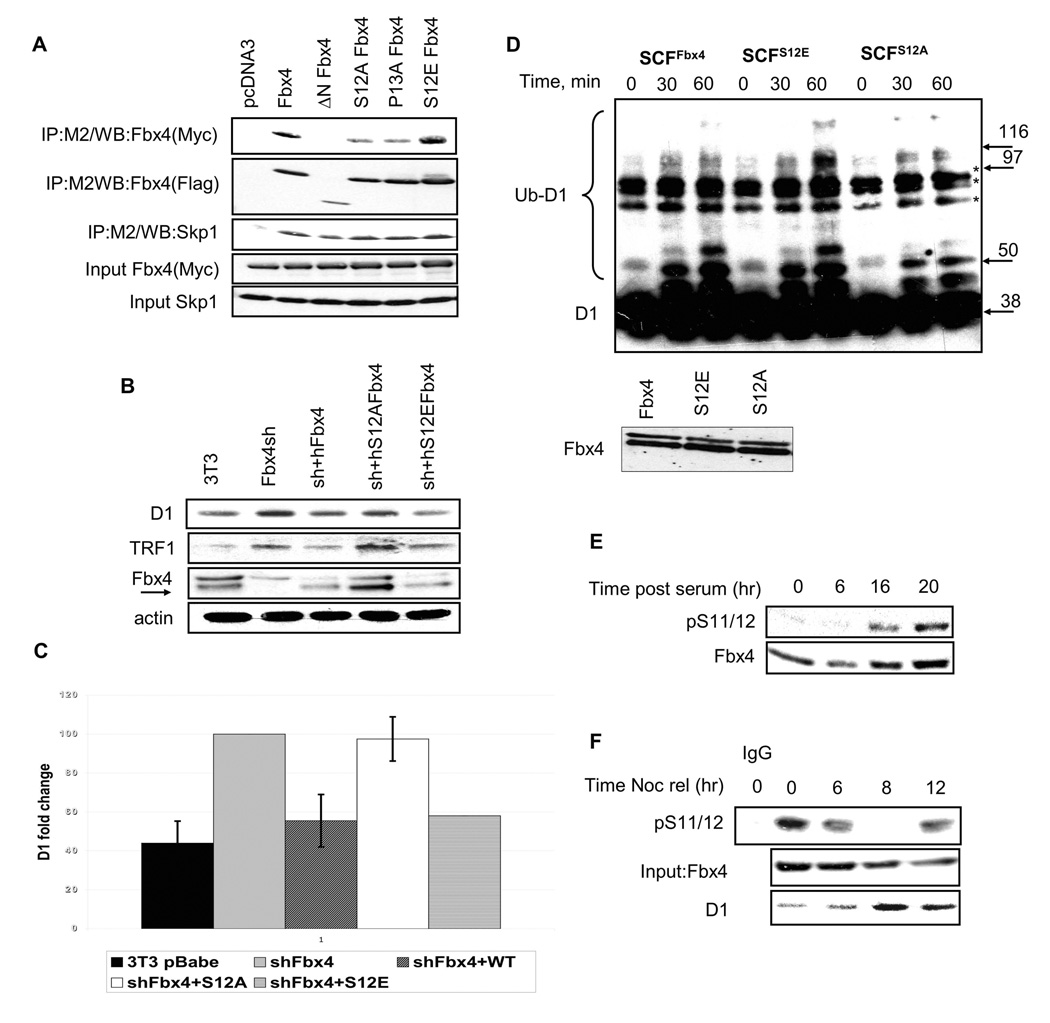
Figure 2. Fbx4 serine 12 is required for Cyclin D1 ubiquitination and proteolysis.
A. 293T cells were transfected with plasmids encoding Flag-tagged Fbx4 mutants and wild type Myc-tagged Fbx4. Complexes were isolated by affinity chromatography using the M2 conjugated agarose and individual components detected by immunoblot with Fbx4 and Skp1 antibodies. B. NIH-3T3 cells, wherein Fbx4 had previously been knocked down by shRNA, were transfected with wt, S12A or S12E Fbx4 pcDNA3 followed by G418 selection of stably expressing clones. Asynchronous cells were harvested and subjected to Western blotting with cyclin D1, TRF1 and Fbx4 antibodies. C. Quantification of B. Error bars indicate +/−SD. D. Ligase complexes (purified protein shown in bottom panel) were purified from stable cell lines expressing wt, S12E and S12A Fbx4 (described in B). In vitro ubiquitination reactions were performed using GST-tagged purified cyclin D1. Ubiquitin-conjugated cyclin D1 was detected by immunoblot. Non-specific complexes are denoted by asterisk. E. NIH3T3 cells were serum starved for 48 hrs and released into 10% FBS containing media for the indicated intervals. Immunoblot analysis was performed using pS11/12-Fbx4 and a total Fbx4 antibody. F. Cells synchronized via a nocodazole block were harvested at the indicated intervals following nocodazole release. Fbx4 phosphorylation was detected by precipitation with the pS11/12 antibody and immunoblot with the total Fbx4 antibody. Total cyclin D1 and Fbx4 levels were assessed by direct western.