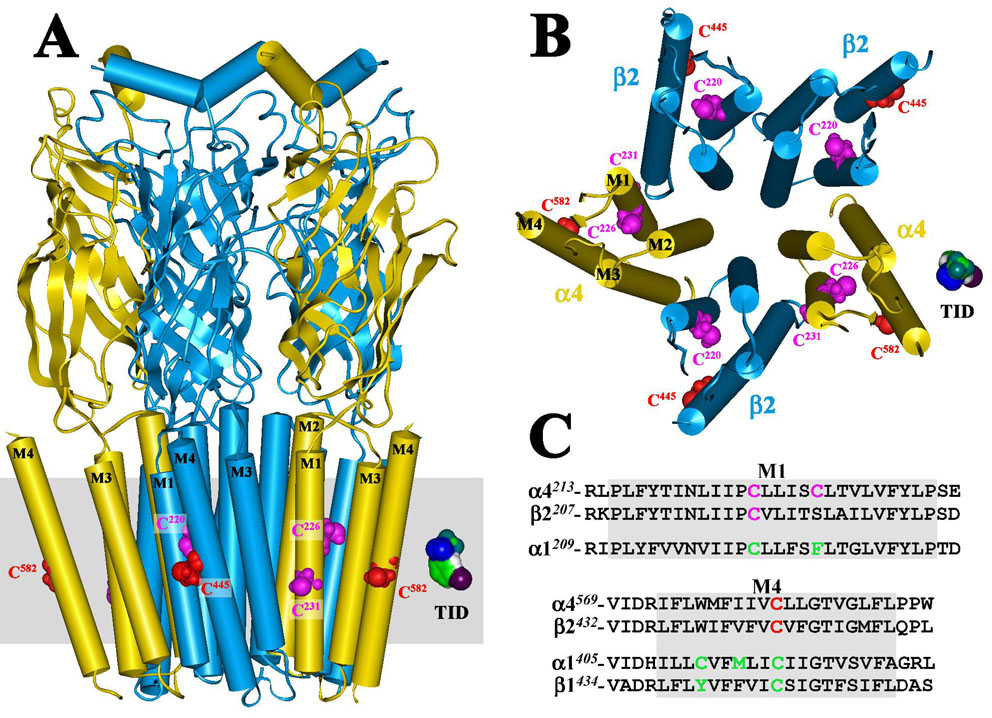
Figure 6. Homology model of the human α4β2 nAChR displaying [125I]TID labeled residues situated at the lipid-protein interface.
A homology model of a human α4β2 nAChR was constructed from the published structure of the Torpedo marmorata nAChR (PDB # 2BG9) as described in the Experimental Procedures. A) A side view of the extracellular and transmembrane domains of the α4 (yellow) β2 (blue) nAChR and B) a view of the transmembrane domain looking through the channel from the synaptic side. The polypeptide chains are traced with regions of α-helix (cylinders) or β-sheet (ribbons) denoted. Residues labeled by [125I]TID are shown in CPK representation within the M1 (magenta) and M4 (red) helices. An approximation of the membrane is included in A (gray) and a Connelly surface model of TID is included for scale. C) An alignment of the M1 and M4 transmembrane helices from human α4 and β2 and Torpedo α1 and β1 (M4 only) nAChRs, with [125I]TID labeled residues highlighted in green (α1M1/M4 and β1M1; ref 12), magenta (M1 of α4 and β2) or red (M4 of α4 and β2).