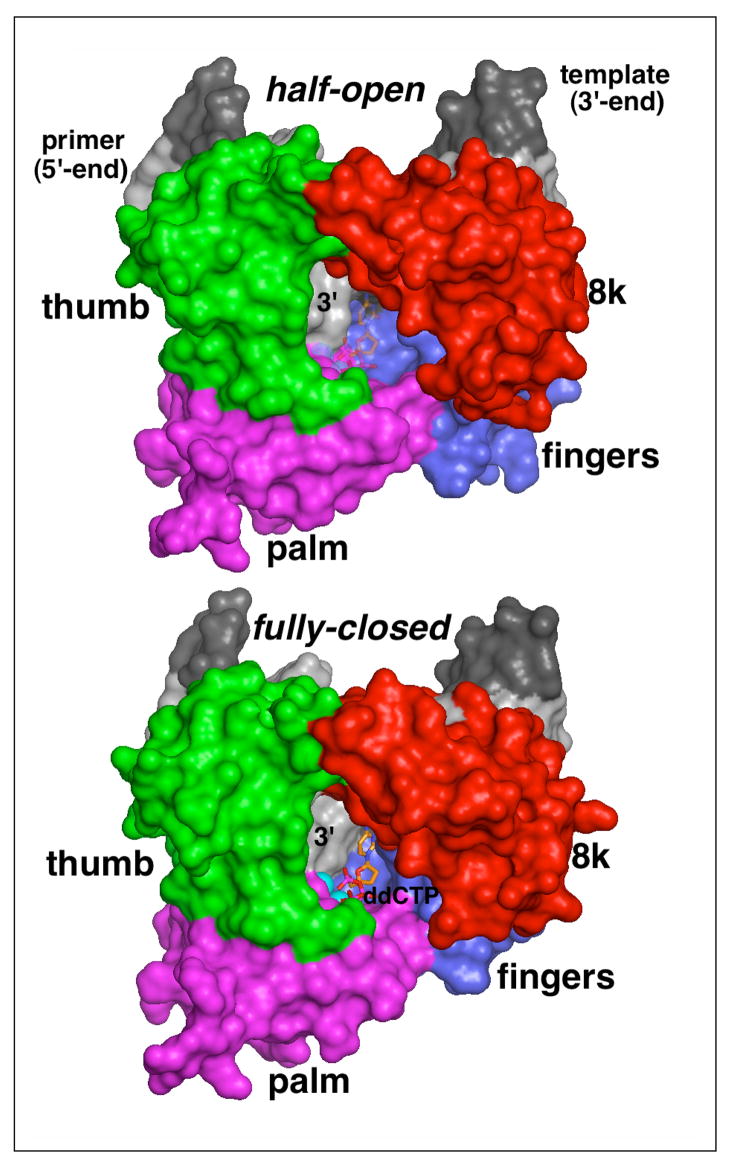
Figure 5. Surface representations of the half-open (top) and fully closed (bottom) form of wild-type polymerase β complexed with gapped DNA.
The surface of polymerase β is colored according to domains (red, 8K; green, thumb; magenta, palm; blue, fingers) and the gapped duplex is shown in shades of gray (dark, template; light, primer). The ddCTP in the half-open form (top), shown as transparent stick model, is modeled into the nucleotide binding pocket by superimposing three nucleotides at the 3′-end of the primer and critical active site residues from the closed conformation onto the respective residues in the half-open form. Note, that the left part of the nucleotide binding pocket is formed by the penultimate nucleotide in the primer strand. In the full-closed form, the fingers domain and the DNA primer have a tight grip around the incoming nucleotide while in the half-open form the geometry and the packing in the dNTP pocket is changed. The apo-polymerase structure of I260Q and computer modeling suggest that the hinge region affects the shape and size of the dNTP pocket, thereby likely leading to changes in the ground-state binding of a mismatched, incoming nucleotide. The turn-over rate of I260Q polymerase β is not affected as the alpha phosphate is still sufficiently close to the 3′-O′ on the primer.