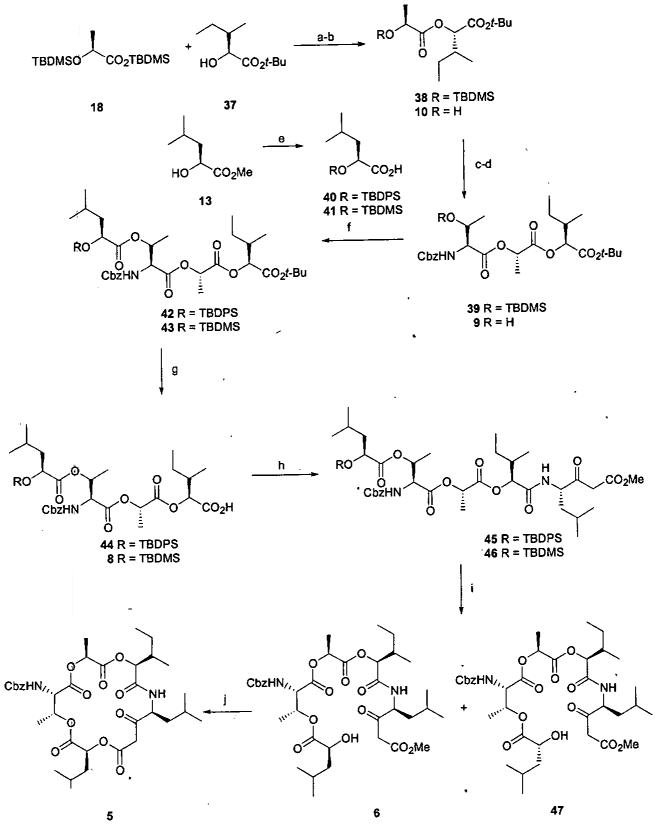
Scheme 6.
a
aReagents and conditions: (a) (i) Oxalyl chloride, catalytic DMF, DCM, 0-23 °C, 2h. (ii) 37, pyridine, 23 °C, 16 h, 81%. (b) TBAF, THF, 23 °C, 1 h, 100%. (c) 25, MNBA, DMAP, TEA, DCM, 23 °C, 16 h, 87%. (d) TBAF, THF, 23 °C, 1 h, 100%. (e) (i) TBDPSCl or TBDMSCl, imidazole, DMF, 23 °C, 16 h. (ii) LiOH, aq THF/CH3OH, 0 - 23 °C, 24 h, 80% for 40, 85% for 41. (f) 40 or 41, MNBA, DMAP, TEA, DCM, 23 °C, 16 h, 85% for 42, 83% for 43. (g) ZnBr2, DCM, 23 °C, 24 h, 80% for 44; SiO2, toluene, 110 °C, 4 h, 59% for 8. (h) (i) 7, 1:1 TFA-DCM, 0.5 h. (ii) PyBroP, DIPEA, DCM, product from (i), 4 h, 52% for 45, 65% for 46. (i) CH3OH, AcCl, 0.5 h, 63%. (j) Toluene, anhydrous CuSO4, 125 °C, 4 h, 80%.