Table 1.
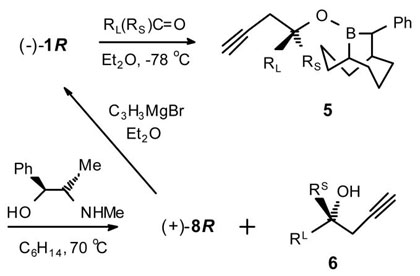
Allenylboration of RLRSCO with 1
 | |||||
|---|---|---|---|---|---|
| RL | RS | 1 | series | 6(%)a | % eeb (abs config) |
| Ph | Me | S | a | 74 | 91 (S) |
| Ph | Me | R | a | 85 | 93 (R) |
| Ph | Et | R | b | 65 | 76 (R) |
| Et | Me | R | c | 71 | 74 (S) |
| Bu | Me | S | d | 80 | 81 (R) |
| i-Pr | Me | R | e | 71 | 84 (R) |
| t-Bu | Me | S | f | 66 | 83 (S) |
| TMS | Me | S | g | 62 | 90 (R) |
| CH2=CH | Me | S | h | 64 | 61 (S) |
The a series was performed with both (−)-1R and (+)-1S. The aS, b, f and h series were conducted employing an oxidative work-up. For the remaining examples, the intermediate 5 was isolated and converted to 6 and either 4 or 8 was recovered (69–81%) via the NMPE work-up procedure.
Product ee determined by conversion to the Alexakis esters6 and analysis by 31P NMR.
