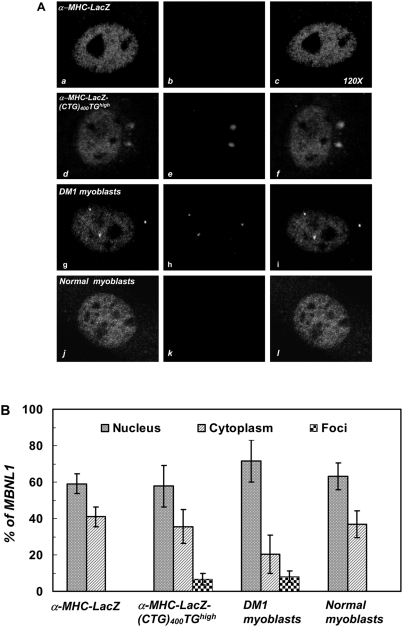
Figure 4. Mbnl1 co-localizes with cytoplasmic CUG-foci in α-MHC-LacZ-(CTG)400 cardiomyocytes.
Panel A: Distribution of endogenous Mbnl1 is visualized as a green signal (a, d, g & j) using anti-MBNL1 (MB1a) monoclonal antibody and secondary antibodies conjugated with FITC. The mutant transcripts encoding the expanded CUG tracts were detected by hybridization with a (CAG)10-Cy3 probe (red signals; e & h). Transcripts containing expanded CUG repeats are not observed in the α-MHC-LacZ cardiomyocytes (b) and normal myoblasts (k). Merged images (f & i), where super-imposition of green and red signals are observed as a yellow signals, show that Mbnl1 co-localizes with the expanded CUG tracts in the α-MHC-LacZ-(CUG)400 cardiomyocytes (f) and in DM1 myoblasts (i). Panel B: Graphical representation of Mbnl1 distribution in each compartment (nucleus, cytoplasm and foci) of α-MHC-LacZ, α-MHC-LacZ-(CUG)400 cardiomyocytes and DM1 and normal myoblasts is shown and the results are tabulated in Table 2. No significant difference is observed in the fraction of Mbnl1 which colocalizes with the foci in α-MHC-LacZ-(CTG)400 cardiomyocytes and in DM1 myoblasts (p = 0.37). The specificity of MBNL1 (MB1a) monoclonal antibody was assessed by immunofluorescence using cardiomyocytes derived from Mbnl1−/− mice (Supplementary Figure S2).