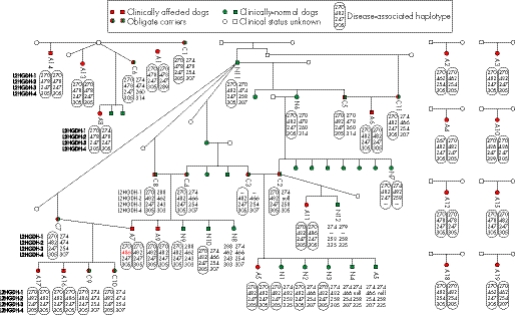
Figure 3 Genotyping data for microsatellites flanking canine L2HGDH in 19 clinically affected (A), 11 obligate carrier (C) and 12 clinically normal but related (N) Staffordshire bull terriers. Clinical status was determined by the presence or absence of l‐2‐hydroxyglutaric acid (l‐2‐HG) accumulation in urine. Clinically affected dogs demonstrated dramatic accumulation and clinically normal dogs demonstrated no to trace accumulation. Obligate carriers demonstrated no l‐2‐HG accumulation, but were dogs that had produced affected offspring or were the offspring of affected animals. The data demonstrate that clinically affected dogs are homozygotic for the disease‐associated haplotype and obligate carriers are heterozygotic, with one disease‐associated haplotype and one wild‐type haplotype. This is consistent with the hypothesis that a mutation in L2HGDH is the cause of l‐2‐HGA in dogs. Clinically normal but related Staffordshire bull terriers had either one or no disease‐associated haplotype. Clinically normal dogs N4 and N5 seemed to be homozygotic for allele 466 of L2HGDH‐2, which is discrepant with the genotypes of their parents at this marker. It is therefore assumed that parent C2 is heterozygotic for allele 482 and for a null allele that fails to amplify, and that N4 and N5 are therefore heterozygotic for 466 and for the null allele. The high mutation rate of canine tetranucleotide microsatellite markers has resulted in an obligate mutation in L2HGDH‐2, from sire and dam C8 and C4, to clinically affected offspring A7 (red allele).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.