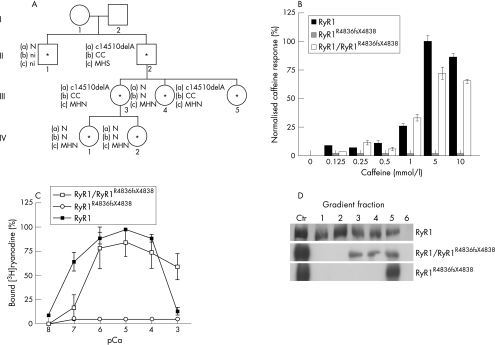
Figure 1 Identification and functional characterisation of the RyR1R4836fsX4838 mutation. (A) The family pedigree. The proband is individual II2. Asterisks indicate individuals who were included in the mutation analysis. (a) Presence (c14510delA) or absence (N) of the mutation. (b) Histological results: central cores (CC), normal (N) and not investigated individuals (ni). (c) In vitro contracture test results. (B) Caffeine responses of human embryonic kidney (HEK)293 cells expressing recombinant RyR1, RyR1R4836fsX4838 and RyR1/RyR1R4836fsX4838 channels. Release was normalised to the maximum Ca2+ release at 5 mM caffeine. Bars indicate mean (standard error of the mean (SEM)) of the percentage of Ca2+ release increments induced by different caffeine concentrations. n = 759 cells for RyR1/RyR1R4836fsX4838; n = 601 cells for RyR1; n = 600 cells for RyR1R4836fsX4838. (C) Ca2+ dependence of [3H]ryanodine binding to microsomal fractions of HEK293 cells with RyR1, RyR1R4836fsX4838 or RyR1/RyR1R4836fsX4838. Mean values obtained from three independent experiments are plotted. Bars indicate the mean (SEM) of 3[H]ryanodine binding. (D) Sucrose density‐gradient analysis of wild‐type RyR1, RyR1R4836fsX4838 and RyR1/RyR1R4836fsX4838 channels expressed in HEK293 cells. As a positive control for RyR1 migration, microsomal vesicles obtained from rabbit skeletal muscle (5 μg) and HEK293 cells expressing RyR1R4836fsX4838 (middle, 30 μg) or RyR1/RyR1R4836fsX4838 (bottom, 30 μg) channels were used (Ctr lane). Fraction 1 represents the bottom of the gradient. MHN, malignant hyperthermia negative; MHS, malignant hyperthermia susceptibility.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.