Table 4.
One-pot tandem reactions; oxidative cross-coupling of 7 with 2-cyclohexen-1-one and Suzuki reaction with various aryl boronic acids.
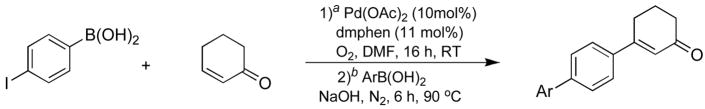 | |||||||
|---|---|---|---|---|---|---|---|
| entry | arylboronic acid | product | yield (%)c | entry | arylboronic acid | product | yield (%)c |
| 1 |

|

|
67 | 6 |

|

|
78 |
| 2 |

|

|
60 | 7 |

|

|
63 |
| 3 |

|

|
51 | 8 |

|

|
30 |
| 4 |

|

|
59 | 9 |

|

|
48 |
| 5 |

|

|
77 | ||||
All reactions were carried out with 4-Iodophenylboronic acid (1.0 mmol), 2-cyclohexen-1-one (2.0 mmol), Pd(OAc)2 (10 mol%), and dmphen (11 mol%)
in DMF (2.5 mL).
Arylboronic acid (1.2 mmol) and NaOH (2.0 mmol).
Isolated yields.
