Abstract
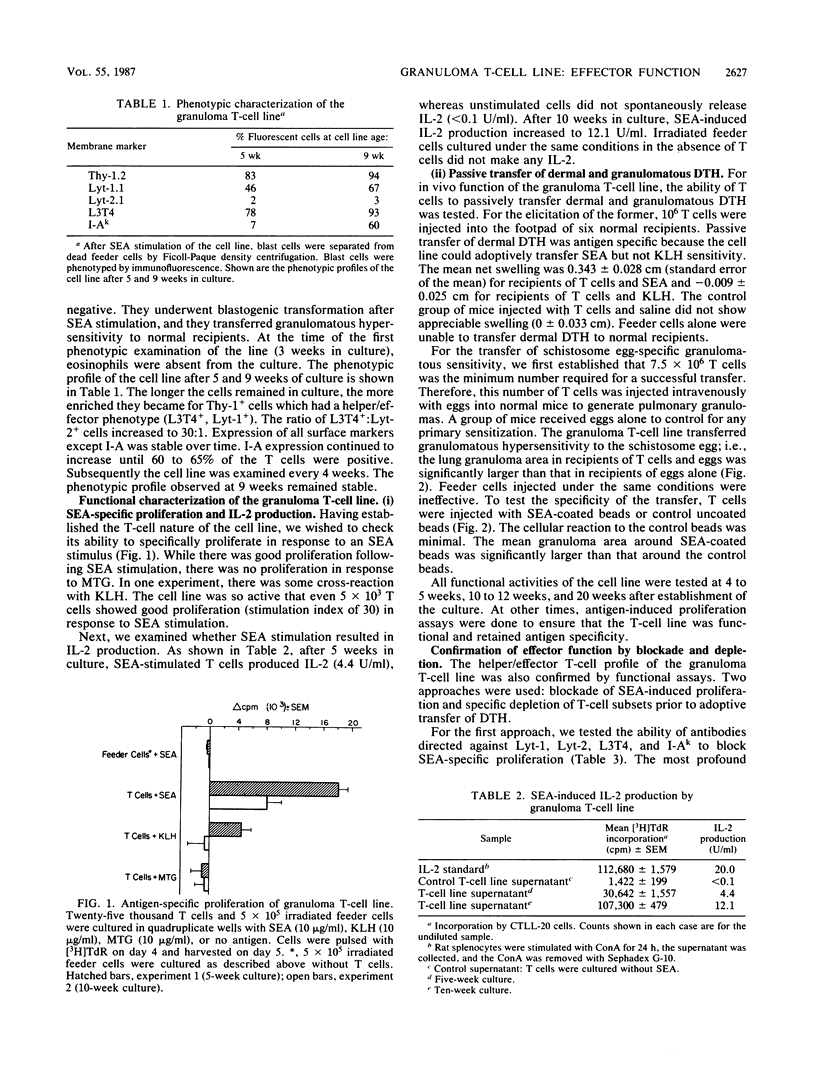
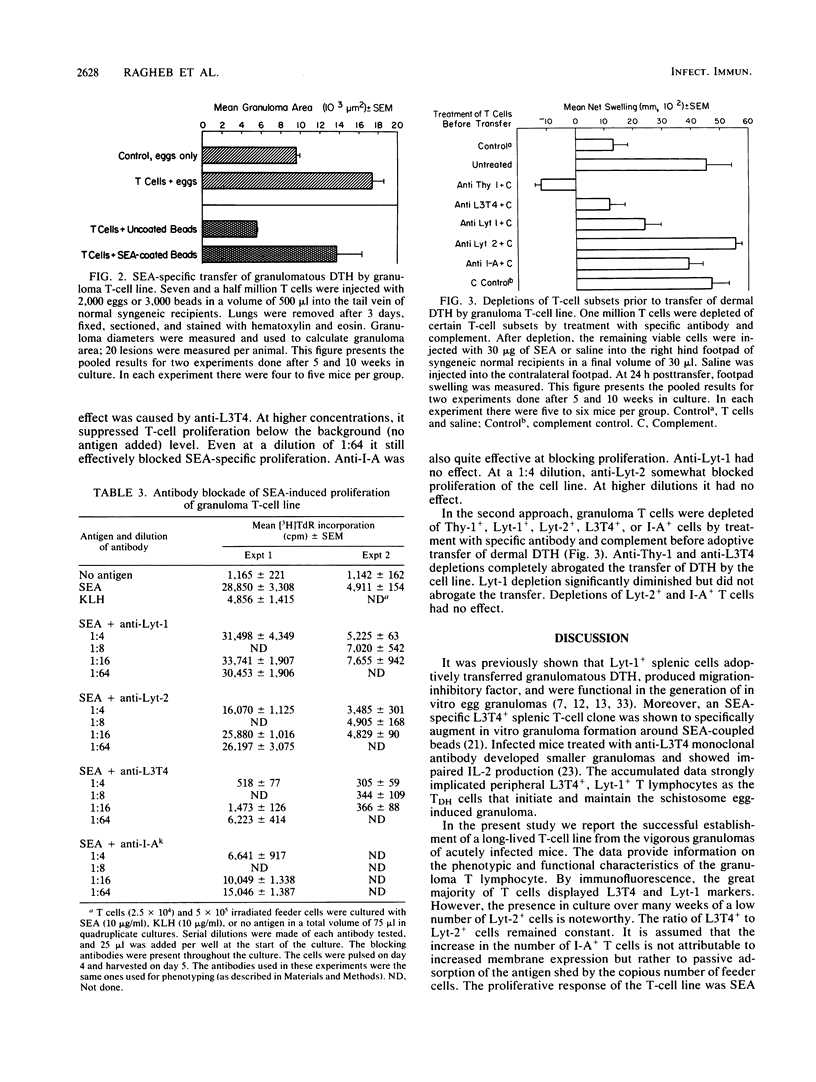
Granulomatous inflammations in schistosomiasis mansoni are the result of T-cell-mediated reactions to soluble egg antigens (SEA) secreted by parasite ova. To study TDH effector cell function, a granuloma T-cell line was established from collagenase-digested liver granulomas of acutely infected CBA/J mice. Dispersed nonadherent granuloma cells were cultured with feeder layer cells and SEA or with feeder layer cells alone in alternate cycles for 32 weeks. The granuloma T-cell line was L3T4+ Lyt-1+. In vitro, the SEA-stimulated T cells showed proliferation and interleukin 2 production. One million T cells adoptively transferred SEA-specific footpad swelling, and 7.5 X 10(6) T cells adoptively transferred granulomatous hypersensitivity to injected ova or SEA-coated beads. Anti-L3T4 monoclonal antibody blocked the SEA-specific cell proliferation. Depletion of L3T4+ cells abrogated, while that of Lyt-1+ cells diminished the adoptive transfer of SEA-specific footpad swelling. These experiments demonstrate that the granuloma T-lymphocyte population contains TDH-type effector cells. Establishment of an SEA-specific granuloma T-cell line will allow the study of the effector functions of the hitherto uncharacterized intralesional granuloma T lymphocyte.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsden A. F., Boros D. L., Hood A. T. Etiology of the liver granulomatous response in Schistosoma mansoni-infected athymic nude mice. Infect Immun. 1980 Jan;27(1):75–80. doi: 10.1128/iai.27.1.75-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L. Experimental granulomatosis. Clin Dermatol. 1986 Oct-Dec;4(4):10–21. doi: 10.1016/0738-081x(86)90030-1. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L., Warren K. S., Pelley R. P. The secretion of migration inhibitory factor by intact schistosome egg granulomas maintained in vitro. Nature. 1973 Nov 23;246(5430):224–226. doi: 10.1038/246224a0. [DOI] [PubMed] [Google Scholar]
- COKER C. M., LICHTENBERG F. A revised method for isolation of Schistosoma mansoni eggs for biological experimentation. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):780–782. doi: 10.3181/00379727-92-22612. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L., David C. S. Regulation of granulomatous inflammation in murine schistosomiasis. In vitro characterization of T lymphocyte subsets involved in the production and suppression of migration inhibition factor. J Exp Med. 1980 Jun 1;151(6):1398–1412. doi: 10.1084/jem.151.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Population dynamics of T and B lymphocytes in the lymphoid organs, circulation, and granulomas of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1979 Mar;28(2):291–299. doi: 10.4269/ajtmh.1979.28.291. [DOI] [PubMed] [Google Scholar]
- Domingo E. O., Cowan R. B., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. I. Immunosuppressive drugs. Am J Trop Med Hyg. 1967 May;16(3):284–292. doi: 10.4269/ajtmh.1967.16.284. [DOI] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. 3. Heterologous antilymphocyte serum. Am J Pathol. 1968 Mar;52(3):613–631. [PMC free article] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. II. Thymectomy. Am J Pathol. 1967 Nov;51(5):757–767. [PMC free article] [PubMed] [Google Scholar]
- Doughty B. L., Phillips S. M. Delayed hypersensitivity granuloma formation and modulation around Schistosoma mansoni eggs in vitro. II. Regulatory T cell subsets. J Immunol. 1982 Jan;128(1):37–42. [PubMed] [Google Scholar]
- Doughty B. L., Phillips S. M. Delayed hypersensitivity granuloma formation around Schistosoma mansoni eggs in vitro. I. Definition of the model. J Immunol. 1982 Jan;128(1):30–36. [PubMed] [Google Scholar]
- Elliott D. E., Boros D. L. Schistosome egg antigen(s) presentation and regulatory activity by macrophages isolated from vigorous or immunomodulated liver granulomas of Schistosoma mansoni-infected mice. J Immunol. 1984 Mar;132(3):1506–1510. [PubMed] [Google Scholar]
- Elliott D. E., Righthand V. F., Boros D. L. Characterization of regulatory (interferon-alpha/beta) and accessory (LAF/IL 1) monokine activities from liver granuloma macrophages of Schistosoma mansoni-infected mice. J Immunol. 1987 Apr 15;138(8):2653–2662. [PubMed] [Google Scholar]
- Grzych J. M., Dissous C., Capron M., Torres S., Lambert P. H., Capron A. Schistosoma mansoni shares a protective carbohydrate epitope with keyhole limpet hemocyanin. J Exp Med. 1987 Mar 1;165(3):865–878. doi: 10.1084/jem.165.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Garrett K. C., Richerson H. B., Fantone J. C., Ward P. A., Rennard S. I., Bitterman P. B., Crystal R. G. Pathogenesis of the granulomatous lung diseases. Am Rev Respir Dis. 1984 Sep;130(3):476–496. doi: 10.1164/arrd.1984.130.3.476. [DOI] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophils and immune mechanisms: production of the lymphokine eosinophil stimulation promoter (ESP) in vitro by isolated intact granulomas. J Reticuloendothel Soc. 1975 Nov;18(5):283–293. [PubMed] [Google Scholar]
- Kaufmann S. H. Biological activities of a murine T-cell clone with reactivity to Mycobacterium leprae. Cell Immunol. 1984 Jan;83(1):215–220. doi: 10.1016/0008-8749(84)90241-7. [DOI] [PubMed] [Google Scholar]
- Lammie P. J., Linette G. P., Phillips S. M. Characterization of Schistosoma mansoni antigen-reactive T cell clones that form granulomas in vitro. J Immunol. 1985 Jun;134(6):4170–4175. [PubMed] [Google Scholar]
- Louis J. A., Zubler R. H., Coutinho S. G., Lima G., Behin R., Mauel J., Engers H. D. The in vitro generation and functional analysis of murine T cell populations and clones specific for a protozoan parasite, Leishmania tropica. Immunol Rev. 1982;61:215–243. doi: 10.1111/j.1600-065x.1982.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Mathew R. C., Boros D. L. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin-2 production in Schistosoma mansoni infection. Infect Immun. 1986 Dec;54(3):820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. L., Grove D. I., Warren K. S. The Schistosoma mansoni egg granuloma: quantitation of cell populations. J Pathol. 1977 Jan;121(1):41–50. doi: 10.1002/path.1711210107. [DOI] [PubMed] [Google Scholar]
- Nickell S. P., Gebremichael A., Hoff R., Boyer M. H. Isolation and functional characterization of murine T cell lines and clones specific for the protozoan parasite Trypanosoma cruzi. J Immunol. 1987 Feb 1;138(3):914–921. [PubMed] [Google Scholar]
- Pelley R. P., Pelley R. J., Hamburger J., Peters P. A., Warren K. S. Schistosoma mansoni soluble egg antigens. I. Identification and purification of three major antigens, and the employment of radioimmunoassay for their further characterization. J Immunol. 1976 Nov;117(5 Pt 1):1553–1560. [PubMed] [Google Scholar]
- Phillips S. M., DiConza J. J., Gold J. A., Reid W. A. Schistosomiasis in the congenitally athymic (nude) mouse. I. Thymic dependency of eosinophilia, granuloma formation, and host morbidity. J Immunol. 1977 Feb;118(2):594–599. [PubMed] [Google Scholar]
- Sheppard H. W., Scott P. A., Dwyer D. M. Recognition of Leishmania donovani antigens by murine T lymphocyte lines and clones. Species cross-reactivity, functional correlates of cell-mediated immunity, and antigen characterization. J Immunol. 1983 Sep;131(3):1496–1503. [PubMed] [Google Scholar]
- Trotter J., Sriram S., Rassenti L., Chou C. H., Fritz R. B., Steinman L. Characterization of T cell lines and clones from SJL/J and (BALB/c x SJL/J)F1 mice specific for myelin basic protein. J Immunol. 1985 Apr;134(4):2322–2327. [PubMed] [Google Scholar]
- Vandenbark A. A., Gill T., Offner H. A myelin basic protein-specific T lymphocyte line that mediates experimental autoimmune encephalomyelitis. J Immunol. 1985 Jul;135(1):223–228. [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]
- Weinstock J. V., Boros D. L. Organ-dependent differences in composition and function observed in hepatic and intestinal granulomas isolated from mice with Schistosomiasis mansoni. J Immunol. 1983 Jan;130(1):418–422. [PubMed] [Google Scholar]


