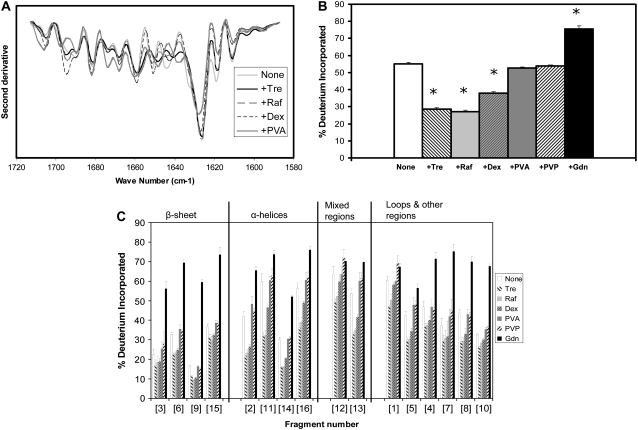
FIGURE 4.
β-Lactoglobulin conformation in amorphous solids containing various additives (1:1 w/w). (A) Second derivative FTIR spectra. (B) ssHDX of intact protein. (C) ssHDX of peptic digests. Additives were Tre, Raf, dex, PVA, PVP, and Gdn. In C, peptic fragments and approximate secondary structures were: 1 (V3-D11, 11% α-helix, 27% turn), 2 (I12-W19, 50% α-helix), 3 (Y20-M24, 100% β-sheet), 4 (I29-V32, 75% turn), 5 (D33-V41, 22% turn), 6 (Y42-L54, 61.5% β-sheet), 7 (L46-L54, 44% β-sheet), 8 (K75-F82, 25% β-sheet, 25% turn), 9 (N90-L95, 83% β-sheet), 10 (D96-L104, 22% β-sheet), 11 (N109-L117, 33% α-helix), 12 (P113-V123, 27% α-helix, 54% β-sheet), 13 (V123-L133, 27% α-helix, 9% β-sheet, 18% turn), 14 (E134-L149, 25% α-helix, 19% β-sheet), 15 (L143-L149, 42% β-sheet), and 16 (S150-L156, 57% α-helix). In B, asterisk (*) indicates significant difference from the value for the “None” sample (α = 0.05); n = 3 ± SD.