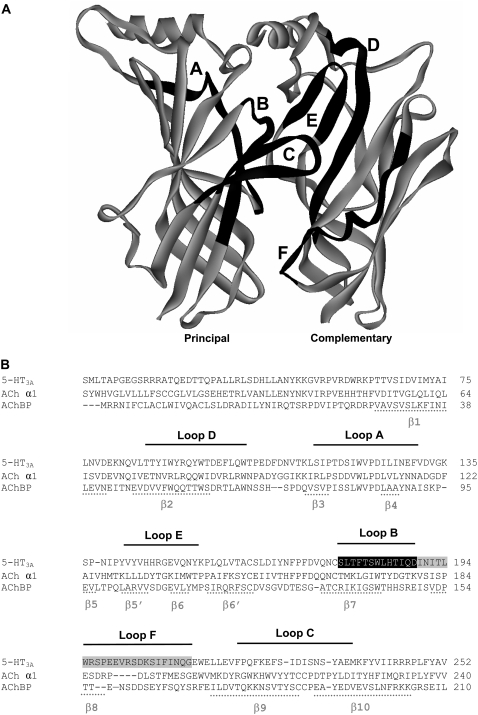
FIGURE 1.
Location of the amino acid residues examined in the current study. (A) Two adjacent subunits (principal and complementary), showing the positions of the six binding loops A-F. (B) The amino acid sequence for the extracellular domain of the murine 5-HT3A receptor (accession No. Q6J1J7), aligned with AChBP isolated from Lymnaea stagnalis (P58154) and the nACh receptor α1 subunit (P02710). The residues examined are highlighted as white text on a black background. Loop F residues that we have reported on previously are highlighted as white text on a gray background (25). The six binding loops are indicated by black lines above the text. The positions of β-sheets are shown by gray lines beneath the text. Numbering of residues and structural features are taken from the AChBP protein crystal structure (3).