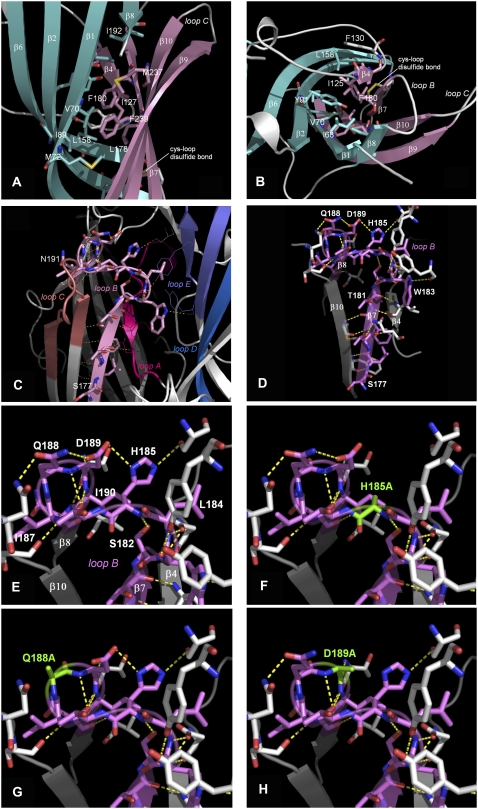
FIGURE 5.
Models of the loop B region of the 5-HT3 receptor. Side (A) and top (B) views of the hydrophobic core of the β-sandwich around Leu-178 and Phe-180. Loop F and a short stretch between the N-terminus and β1 have been omitted for clarity. Inner β-sheets in aquamarine, outer β-sheets in pink. Hydrophobic residues within 5 Å of Leu-178 and Phe-180 are rendered in stick representation and color coded according to the corresponding β-sheets. This hydrophobic core includes Ile-68, Val-70, Met-72, Leu-85, Ile-89, Tyr-91, Ile-125, Ile-127, Phe-130, Leu-158, Leu-178, Phe-180, Ile-192, Met-237, Phe-239, and Val-241. The hydrophobic nature of these residues is conserved across the Cys-loop family (see Fig 1). (C) The distribution of potential hydrogen bonds between loop B and adjacent regions. (D) Hydrogen bonds between residues within loop B. Hydrogen bonds that may stabilize the β7-β8 turn are shown in E, and mutation of these residues (F–H) could disrupt this pattern. Hydrogen bonds in this figure were predicted, and are visualized, using PyMOL v 0.98.