Abstract
Human eyes are a powerful social cue that may automatically attract the attention of an observer. Here we tested whether looking towards open human eyes, as often arises in standard clinical “confrontation” tests, may affect contralesional errors in a group of right brain-damaged patients showing visual extinction. Patients were requested to discriminate peripheral shape-targets presented on the left, right or bilaterally. On each trial they also saw a central task-irrelevant stimulus, comprising an image of the eye-sector of a human face, with those seen eyes open or closed. The conditions with central eye stimuli open (versus closed) induced more errors for contralesional peripheral targets, particularly for bilateral trials. These results suggest that seeing open eyes in central vision may attract attentional resources there, reducing attention to the periphery, particularly for the affected contralesional side.
The seen gaze of the examiner may thus need to be considered during confrontation testing, and may contribute to the effectiveness of that clinical procedure.
Introduction
Right brain-damaged patients often show inattention to contralesional stimuli either delivered in isolation (neglect) or accompanied by concurrent ipsilesional stimuli (extinction). This may reflect pathologically biased and restricted attentional capacity (1), and is typically tested for using clinical ‘confrontation’. The examiner faces the patient and moves his/her own fingers on one side (unilateral stimuli) or both sides (bilateral) of the patient's peripheral visual field. The patient typically has to look straight at the examiner's eyes or nose, while reporting any seen finger movement. The examiner likewise fixates the patient directly, to ‘confront’ his/her visual field against the patient's. In this clinical setting, the patient sees the examiner's face gazing directly at them in central vision, while being required to judge peripheral stimuli. This aspect of the confrontation procedure might actually modulate processing of peripheral stimuli, since direct gaze can be a potent social stimulus engaging attention (2-4).
While averted gaze in the seen display has been shown to modulate inattention for contralesional stimuli (5), to our knowledge the possible impact of seeing direct gaze (versus closed eyes) has not been addressed. We hypothesized that when patients see open eyes gazing at them in central vision, this will engage attentional resources centrally, to the detriment of peripheral targets, especially on the affected (contralesional) side. We first encountered this phenomenon clinically in a single-case, who showed reliably more left extinction during conventional confrontation, when the examiner gazed direct at the patient's nose, or gazed down (showing 87% vs. 25% extinction respectively). We followed this up with a controlled experiment, presenting computerized visual targets on the left, right, or both sides, together with central images of human eyes that were either open (with direct gaze) or closed.
Methods
Six right-handed, right brain-damaged patients (Table 1) and six healthy controls (mean age 58.5 vs. 63.7 years, p>.05) consented to participate. Patients were selected by presence of unilateral right brain damage for two months or more before the experiment; ability to comply with task instructions, particularly in maintaining central fixation (as monitored by the experimenter); and presence of visual extinction. Extinction was confirmed with a preliminary computerized test. Patients fixated a central cross and judged whether small blue triangles appearing for 100 milliseconds at 15 degrees of visual angle to the left or right (unilateral trials, 6 per side) or on both sides (12 bilateral trials), each pointed upwards or downwards (Figure 1). This 2 AFC task provides a sensitive measure of any contralesional deficit.
TABLE 1.
Demographic and screening details of the patients group.
| Patient | Age |
Lesion site (right hemisphere) |
Months from stroke |
Visual field defect |
Star cancellation test |
Line bisection | Tactile ext |
Visual ext (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | F, T, P | 5 | − | 22 | 1 | + | 25 |
| 2 | 64 | F, Pv-WM, IC | 4 | LIQ | 5 | 6 | + | 50 |
| 3 | 65 | P | 8 | − | 0 | −1 | − | 17 |
| 4 | 56 | F-P | 12 | − | 0 | 0 | − | 33 |
| 5 | 80 | F-T | 9 | − | 0 | 2 | − | 17 |
| 6 | 67 | BG | 18 | − | 0 | 1 | + | 17 |
Table legend: Lesion site: F= Frontal; T= Temporal; P= Parietal, PV-WM= Periventricular white matter; IC= Internal capsule; BG= Basal ganglia. All lesions were located in the right hemisphere. Visual field defect: LIQ= Left inferior quadrantanopia. Star Cancellation: number of stimuli omitted on the left side (6). Line bisection: Rightward shift (in millimeters) on the bisection of a 20 cm-long line. Tactile extinction: Tested clinically with light touches on the dorsal aspect of the hand (+ = present; − = absent). Visual extinction: Left errors on bilateral trials at the computerized screening test (see text for details and figure 2A for detailed results).
Figure 1. Stimulation display.
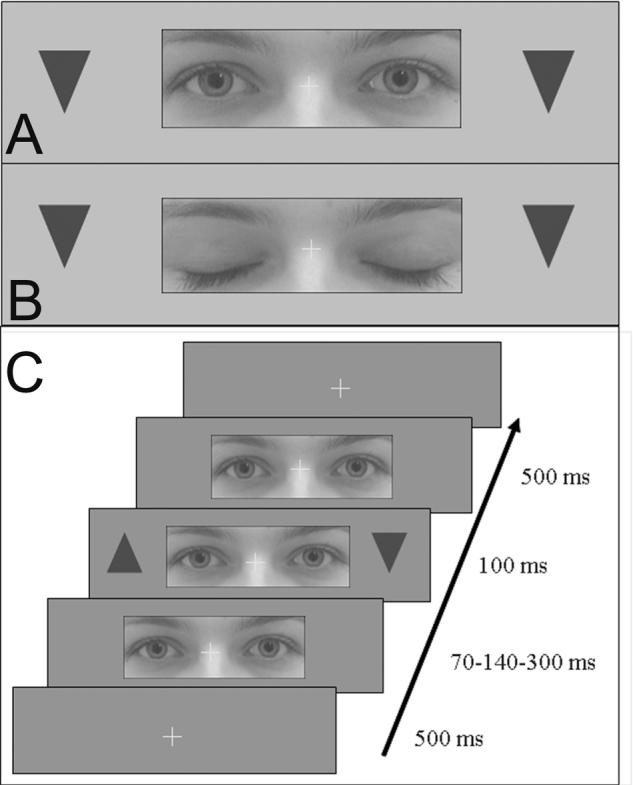
In the screening and experimental test, patients judged the orientation of triangular shapes appearing on right or left of a fixation cross. In the experimental test only, target stimuli were preceded by a central photograph of female human eyes, which could be either eyes-open (A) or eyes-closed (B). The time course of stimulus presentation is shown in (C).
The subsequent experimental phase comprised identical peripheral targets, plus a central ‘distractor’ comprising a picture of the eye-region from a human face (size: 12×4.5 degrees) overlapping and centered on the central fixation cross, appearing for a variable interval (70, 140, or 300 ms, to minimize systematic anticipation) prior to the peripheral target(s). Critically the eyes of the central face could be open (with direct gaze), or closed (Fig. 1A-C). Patients fixated the central cross and gave discriminative responses to the triangle targets (up or down, for any side stimulated). Verbal responses were recorded. Each block comprised 24 unilateral (12 right, 12 left) and 24 bilateral stimuli; half of the trials had the centrally-depicted eyes open, the other half had these closed, in a random sequence. Patients #3-6 completed three blocks, patients #1-2 only two blocks. We predicted that errors on contralesional targets should increase with an eyes-open as compared to eyes-closed central distractor.
Results
On initial screening (Figure 2A), patients committed more left errors on bilateral trials (extinction), while some errors was noted for unilateral left trials also. The latter probably reflect some degree of neglect (6) or residual neglect in our patients (see Table 1). Some errors were made even on the right, due to the brief display and demanding discrimination.
Figure 2. Results.
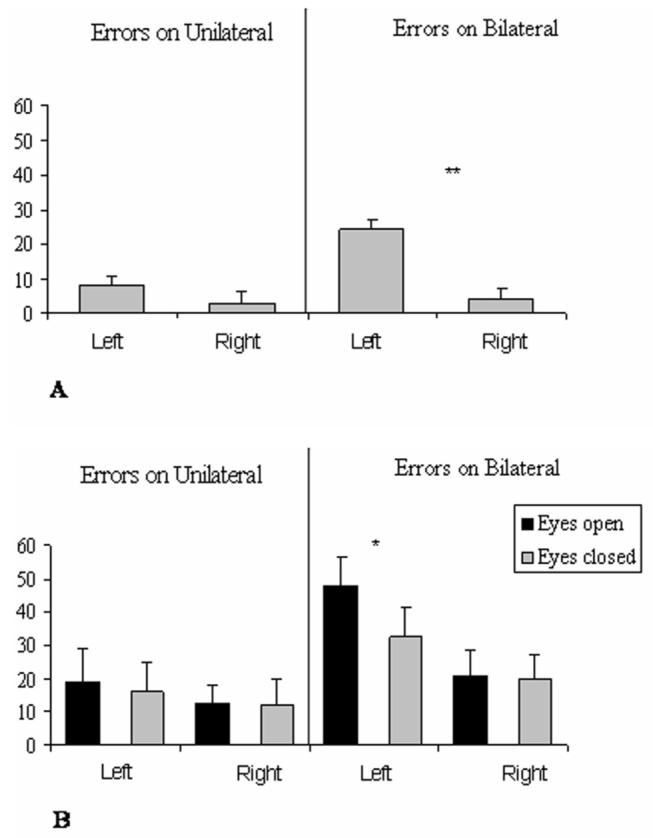
Percentage of errors in the baseline (A) and Experimental (B) sessions, committed on each side on unilateral and bilateral trials. For bilateral trials, errors could be made on the left or right side, or bilaterally, thus data for right and left errors are presented separately. Errors on the left side were either omissions (45%) or incorrect discriminations (55%); any errors on the right side were wrong discriminations. Error bars indicate standard error, asterisks indicate a difference with p<.01.
For the experimental results (Figure 2B), an analysis of variance (ANOVA) was performed on the percentage of errors with three within-subject factors: SOA (70-140-300 milliseconds); stimulus-number (unilateral, bilateral); error-side (left, right); distractor-eyes (open, closed).
Results showed significantly more discrimination errors on bilateral than unilateral trials (30% vs.15%); more errors on the left than right (29% vs. 16%), and an interaction between these factors (F(1,5)=15, p=.011), due to a bigger effect of side for bilateral (40% vs. 21%) than unilateral trials (18% vs. 11%), consistent with left extinction (6).
Importantly, an interaction between Error-side and Distractor-eyes was found (F(1,5)=8,2; p<.05), with an eyes-open central distractors leading to more errors (33% vs. 25%) for left targets, but not right targets (17% vs. 15%). Figure 2 shows that this was most pronounced for bilateral trials (48% vs. 32%, t(5) =4.9, p<.01), while only a small trend was also found for left unilateral targets (possibly reflecting an influence of the distractor eyes on neglect, or extinction of a left target by the central eyes; see also 5, their experiments 4 and 5).
There was only a non-significant tendency for more errors in the eyes-closed distractor condition than the baseline without a distractor, further suggesting that the open eyes have the biggest distractor impact.
Discussion
Errors in the contralesional hemifield worsened when the distractor at central fixation had (direct) eyes open rather than closed. Seeing a face looking straight towards us in central vision may attract attention (2), draining this from more peripheral locations, to the detriment of the disrupted (contralesional) peripheral field in right brain-damaged patients. We found no evidence that the central open-eyes could improve left performance (as might have been expected instead, if drawing attention to the center can remediate the patient's rightward bias). Instead, the opposite pattern of worse left performance was observed.
Our finding may relate to clinical confrontation tests. The examiner's own eyes may analogously attract central fixation when gazing directly at the patient with open lids, thereby potentially increasing contralesional inattention. Indeed, we confirmed this clinically in a single case (see Introduction). Natural social attention cues, from gaze between examiner and patients might thus contribute to making the simple clinical test particularly sensitive, and so should be considered when implementing this in the clinic.
Acknowledgements
This research was funded by grants from the Italian MURST (AM); the Medical Research Council (UK) and the Wellcome Trust (JD and MH); and the Swiss National Science Foundation (PV and SS). The authors thank Nathalie George and Michela Avella for technical assistance, and Paola Ricciardelli for helpful discussion.
Footnotes
Disclosure: The authors have reported no conflicts of interest
References
- 1.Driver J, Mattingley JB, Rorden C, Davis G. Extinction as a paradigm measure of attentional bias and restricted capacity following brain injury. In: Thier P, Karnath H-O, editors. Parietal lobe contributions to orientation in 3D space. Springer Verlag; Heidelberg: 1997. pp. 401–429. [Google Scholar]
- 2.Senju A, Hasegawa T. Direct gaze captures visuospatial attention. Visual Cognition. 2005;12:127–144. [Google Scholar]
- 3.George N, Driver J, Dolan RJ. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- 4.Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science. 2004;15:598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 5.Vuilleumier P. Perceived gaze direction in faces and spatial attention: a study in patients with parietal damage and unilateral neglect. Neuropsychologia. 2002;40:1013–1026. doi: 10.1016/s0028-3932(01)00153-1. [DOI] [PubMed] [Google Scholar]
- 6.Bisiach E, Vallar G. Handbook of Neuropsychology. 2 ed Elsevier; Amsterdam: 2000. Unilateral neglect in humans; pp. 459–502. [Google Scholar]


