Figure 7.
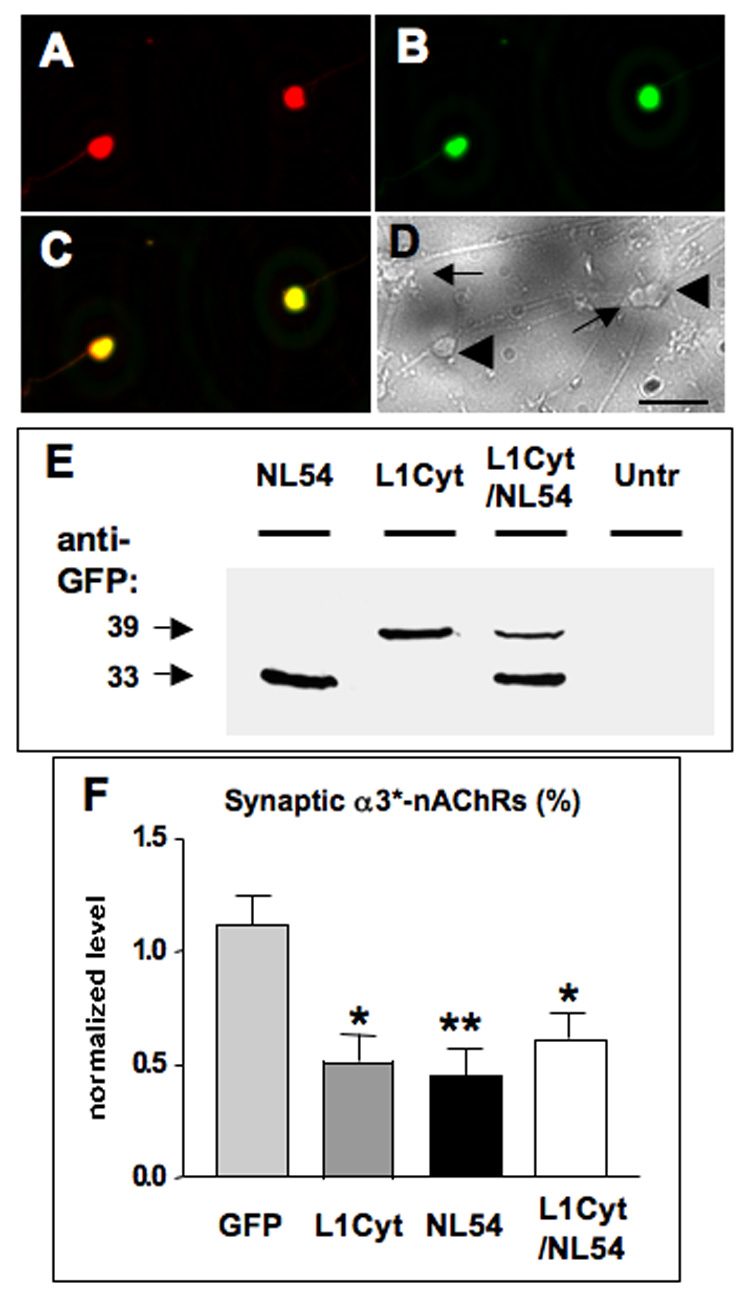
Simultaneous L1 and NL disruption indicates that both are necessary for the same population of synapses. CG neurons in culture were transfected with a construct encoding both L1Cyt-GFP and NL54-GFP (L1Cyt/NL54) and then immunostained with a specific anti-human L1 antibody to discriminate between L1Cyt and endogenous chicken L1. The L1 antibody recognized (A) the L1Cyt peptide (red) in each (B) GFP-expressing cell (green), seen (C) in the overlay (yellow), but not (D) in any untransfected cells as seen in a brightfield image from the same field of view (untransfected cells, arrows; transfected cells, large arrowheads). (E) Western blots of culture lysates probed for GFP to reveal NL54-GFP and L1Cyt-GFP (33 and 39 kDa, respectively), confirming that both are expressed from the L1Cyt/NL54 construct. Untransfected cultures showed no bands (Untr), confirming specificity. Similar results were obtained in a second experiment. Electroporation of CG neurons in ovo followed by image analysis indicated that expression of L1Cyt, NL54, or L1Cyt/NL54 each significantly reduced the proportion of α3*-nAChR clusters overlaid with SV2 puncta. (F) The reduction caused by L1Cyt/NL54 is quantitatively indistinguishable from similar reductions produced by either L1Cyt or NL54 alone (from Fig 5B). *p < 0.05; **p < 0.01 compared to GFP by ANOVA with Bonferroni post-tests. Cell numbers for GFP, L1Cyt, and NL54 were as in Fig. 4; 22 cells were analyzed for the L1Cyt/NL54 construct (1–4 cells per ganglion), plus 2–4 unelectroporated control cells for each electroporated cell in the same section. Scale bars: 40 µm.
