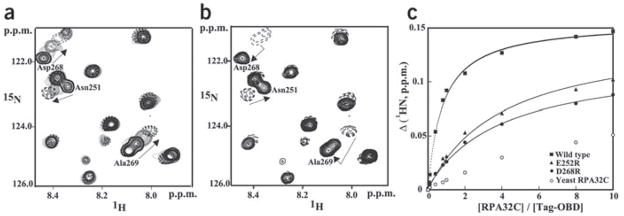
Figure 3.
Effects of DNA binding and mutations on the interaction between hRPA32C and Tag-OBD. (a,b) Comparison of the binding of Tag-OBD to hRPA32C in the absence (a) and presence (b) of origin DNA. Unlabeled Tag-OBD was titrated into a 100 mM solution of 15N-enriched hRPA32C and a series of 15N,1H HSQC NMR spectra were acquired. An overlay of a small region from these spectra is shown in a. A stoichiometric amount of origin DNA duplex was then titrated into the solution and an additional spectrum was acquired (b). Arrows facilitate following the change in the location of the NMR signal. (c) NMR 1H chemical shift titration curves for the binding of wild-type, E252R, E268R and yeast hRPA32C to 15N-labeled Tag-OBD. The changes in amide proton chemical shifts of Thr199 are plotted against the ratio of Tag-OBD to hRPA32C. The line through each curve represents a best fit to the standard single-site binding equation.