Figure 2. Thrombopoietin reduces c-Myb protein and function in UT7/TPO cells and in murine megakaryocytes.
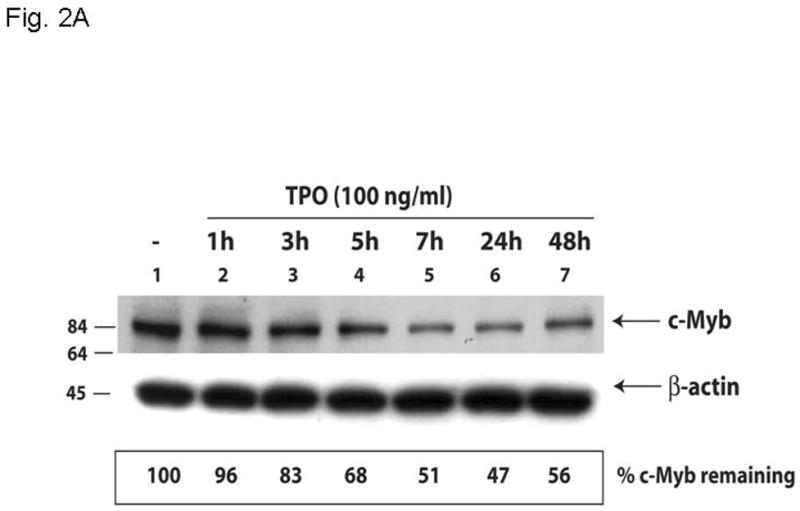
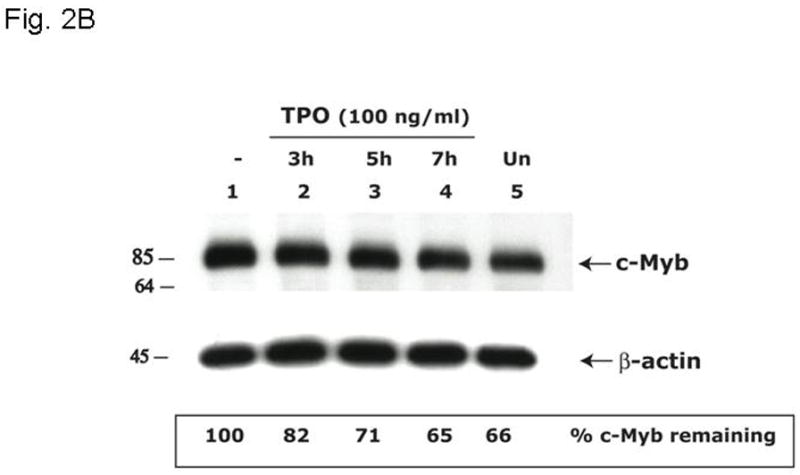
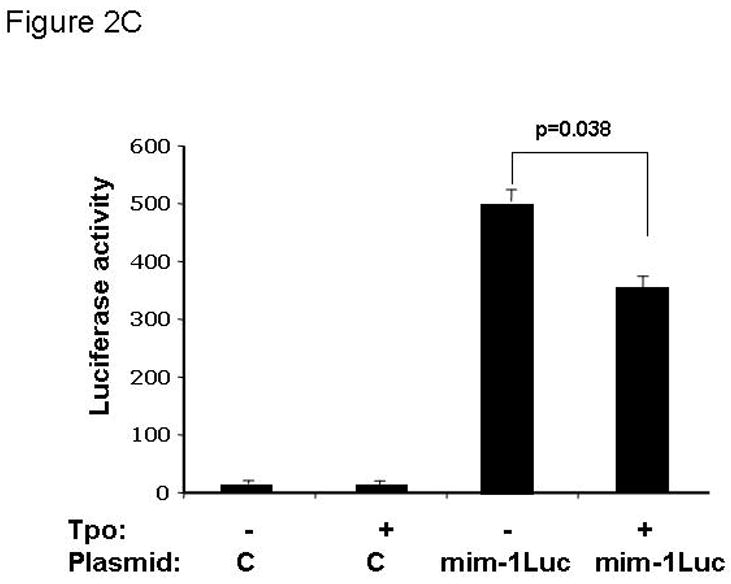
A. UT7/TPO cells were starved overnight and then stimulated with 100 ng/ml thrombopoietin for 0–48 hr, cell lysates were prepared, size fractionated, transferred to PVDF membranes and probed for c-Myb. Densitometric analysis revealed a significant decrease in c-Myb protein levels at 5h–48h (p≤0.01 for all time points). The fraction of c-Myb remaining is shown, along with a representative western blot. B. Mature murine megakaryocytes were produced by marrow cell culture in thrombopoietin and the cells purified by BSA gradient and starved for 20 hr in StemPro serum-free medium. The cells were then cultured in 100 ng/ml thrombopoietin for up to 7 hr, cell lysates prepared and subjected to western blotting for c-Myb and β-actin,. Densitometric analyses (n=3) showed c-Myb protein levels were significantly decreased at 5–7h (p≤0.01). The fraction of c-Myb remaining is shown, along with a representative western blot. C. The c-Myb responsive reporter construct c-mim-luc (mim1Luc), which contains three c-Myb response elements was introduced into UT7/TPO cells and following overnight starvation was cultured an additional 5 hr with thrombopoietin or control culture medium. Sham transfected cells served as a control (C). The data represent the mean of three experiments. The difference between thrombopoietin and no thrombopoietin in c-mim1-luc transduced cultures achieved statistical significance (p=0.038).
