Table 3.
Substrate scope under optimized conditions.
| entry | product | mol% cat.a | temp (°C)/time (h) | % yield (% ee) | |
|---|---|---|---|---|---|
| 1 |
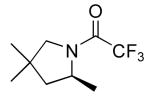
|
2 | 20 | 115/24 | 95(80)c |
| 10 | 115/48 | 91(80)c | |||
| 2 |
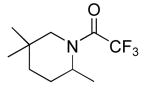
|
14 | 20b | 85/24 | 99(51)c |
| 10b | 85/48 | 91(51)c | |||
| 3 |
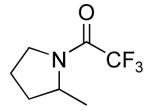
|
15 | 20 | 135/72 | 33(62)c,d |
| 4 |
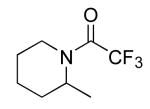
|
16 | 10 | 135/24 | 79(33)c |
| 5 |
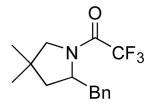
|
17 | 20 | 135/24 | 93(62)c |
| 6 |
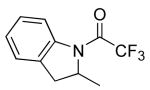
|
18 | 20 | 115/48 | 93(70)c |
| 20 | 115/48 | 85(70)e | |||
| 7 |
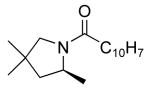
|
19 | 10 | 115/48 | 78(80)f |
| 8 |
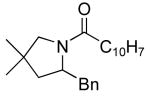
|
20 | 20 | 135/24 | 75(55)f |
Reactions were performed in toluene.
Prepared in situ by combination of equimolar amounts of ligand and Zr(NMe2)4 in toluene and heating at the reaction temp for 15 min prior to introduction of substrate.
Ligand and Zr(NMe2)4 pre-heated at 105 °C for 15 min.
GC yield determined using C6Me6 as an internal standard; ee determined by chiral GC.
32% starting material remained in solution at the end of the reaction.
Isolated yield; ee determined by chiral GC.
Isolated yield as 2-napthoyl amide; ee determined by chiral HPLC.
