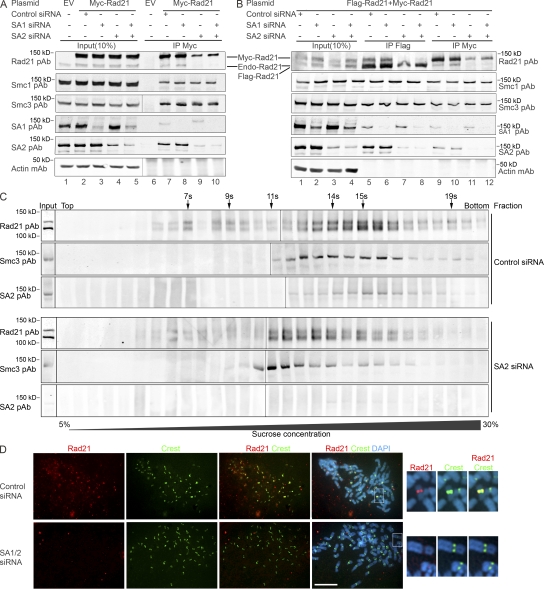
Figure 6.
Knockdown of SA1/SA2 disrupts the Rad21–Rad21 interaction and the formation of the two-ring cohesins. (A and B) SA1 and SA2 are knocked down by respective siRNA 24 h after transfection with tagged Rad21 into 293T cells. (A) Myc-Rad21 cannot coimmunoprecipitate endogenous Rad21 when SA2 is knocked down. (B) Myc-Rad21 and Flag-Rad21 cannot reciprocally coimmunoprecipitate each other when SA2 is down-regulated. (C) Sucrose gradient centrifugation to study cohesin–cohesin interaction after SA2 knockdown. 293T cells were transfected with SA2 siRNA or control siRNA, and, 24 h later, the cells were cotransfected with Myc-Rad21 and Flag-Rad21 for 40 h. Cell lysates were prepared and used in sucrose gradient centrifugation. Rad21, Smc3, and SA2 were analyzed using WB. Sedimentation coefficient is shown on the top of the blot. Input, sample before sucrose gradient centrifugation. (D) Dissociation of cohesin from sister chromatids in SA2 knockdown cells. HeLa cells in the mitosis phase were cytospun onto slides, and immunofluorescent microscopy was performed. Rad21 mAb and human CREST antibodies were used to probe Rad21 (red) and centromere (green), respectively. The nuclear material is visualized by DAPI staining (blue). The centromeres of one chromosome shown in the boxes of merge panels are enlarged on the right. (A and C) Black lines indicate that intervening lanes have been spliced out. Bar, 10 μm.