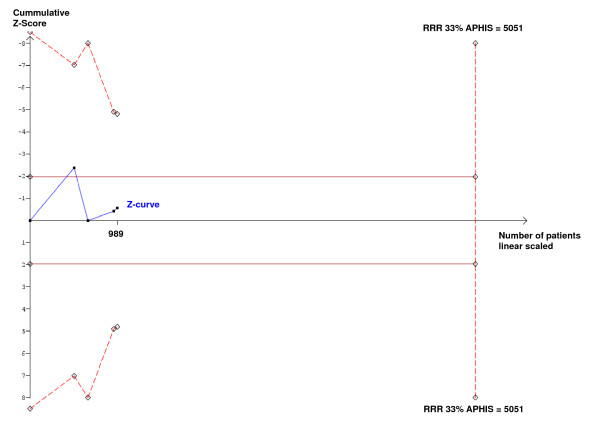
Figure 2.
Trial sequential analysis with a required information size of 5051. A priori heterogeneity adjusted information size (APHIS) based on an a priori relative risk reduction (RRR) of 33% with a type I error risk of 5% and a power of 80%. The cumulative z-curve constructed for a random effects model as heterogeneity is 74% crosses the traditional boundary (P = 0.05) once and return to non-significant values. The cumulative z-curve never crosses the trial sequential monitoring boundary. Despite 989 patients randomized we may still need more than 4000 randomized participants to close the information gap considering repeated analyses of accumulating data.