Abstract
When given systemically to rats and humans, the drug of abuse 3–4 methylenedioxymethamphetamine (ecstasy, MDMA) elicits hyperthermia, hyperactivity, tachycardia, and hypertension. Chemically stimulating the dorsomedial hypothalamus (DMH), a brain region known to be involved in thermoregulation and in stress responses, causes similar effects. We therefore tested the hypothesis that neuronal activity in the DMH plays a role in MDMA-evoked sympathetic and behavioral responses by microinjecting artificial CSF or muscimol, a neuronal inhibitor, into the DMH prior to intravenous infusion of saline or MDMA in conscious rats. Core temperature, heart rate, mean arterial pressure and locomotor activity were recorded by telemetry every minute for 120 minutes. In rats previously microinjected with CSF, MDMA elicited significant increases from baseline in core temperature (+1.3 ± 0.3°C), locomotion (+50 ± 6 counts/min), heart rate (+142 ± 16 beats/min), and mean arterial pressure (+26 ±3 mmHg). Microinjecting muscimol into the DMH prior to MDMA prevented increases in core temperature and locomotion and attenuated increases in heart rate and mean arterial pressure. These results indicate that neuronal activity in the DMH is necessary for the sympathetic and behavioral responses evoked by MDMA.
Keywords: 3,4-Methylenedioxymethamphetamine; Hypothalamus; Thermoregulation; Behavioral; Cardiovascular; Muscimol
2. Introduction
The popular drug of abuse 3,4-methylenedioxymethamphetamine (ecstasy or MDMA) is associated with numerous medical complications (Gowing et al., 2002). In both humans and laboratory animals, MDMA evokes complex physiologic and behavioral responses including, but not limited to, increases in locomotion (Spanos and Yamamoto, 1989), increases in heart rate and blood pressure (Dumont and Verkes, 2006; Rusyniak et al., 2007), and hyperthermia (Dumont and Verkes, 2006; Gowing et al., 2002). MDMA is thought to mediate these responses by facilitating monoaminergic neurotransmission. Indeed, numerous studies suggest that the responses evoked by MDMA involve receptors for serotonin, dopamine, and norepinephrine (Aguirre et al., 1998; Callaway et al., 1992; Green et al., 2004; Herin et al., 2005; Rusyniak et al., 2007; Sprague et al., 2004).
Despite our current understanding of its pharmacology, the central sites and mechanisms by which MDMA evokes its responses are unknown. In part, this is due to complex interactions between neurotransmitters affected by MDMA and the receptors through which these neurotransmitters act. One strategy to overcome these difficulties, and the one this paper employs, is to first identify central sites involved in MDMA’s effects. Once identified, characterizing the relevant neurons in these regions may provide insights into new treatment strategies. To date, however, the specific brain regions involved in mediating MDMA’s effects are largely unknown. The dorsomedial hypothalamus (DMH), classically, considered part of the “hypothalamic defense area”, plays an essential role in responses to physiologic stress and experimental fever (DiMicco et al., 2002; Zaretskaia et al., 2002). When excited or disinhibited, neurons in the DMH evoke tachycardia, hypertension, hyperthermia and increased locomotion in rats (Cao et al., 2004; DiMicco et al., 2006; Zaretskaia et al., 2002) -- effects strikingly similar to those produced by systemic doses of MDMA. Conversely, microinjecting muscimol, a GABAA agonist and neuronal inhibitor, into the DMH suppresses tachycardia and hyperthermia elicited by either restraint stress or preoptic microinjections of prostaglandin E2 (DiMicco et al., 1996; Zaretskaia et al., 2003).
We hypothesized that physiologic and behavioral effects mediated by the systemic administration of MDMA depend on neuronal activity in the DMH. This study’s principal aim was to assess the effects of microinjecting muscimol into the DMH on the thermogenic, cardiovascular, and behavioral responses evoked by subsequent intravenous injection of MDMA in conscious rats.
3. Results
Baseline Physiologic parameters
Baseline parameters for temperature in degrees Celsius (°C), locomotion in activity units (a.u.), heart rate in beats per minute (beats/min) and mean arterial pressure in millimeters of mercury (mmHg) were similar in all groups (table 1).
Table 1.
Baseline values for temperature, locomotion, heart rate, and mean arterial pressure in each treatment group.
| n | Temp (°C) | Locomotion (a.u.) | Heart Rate (bpm) | MAP (mmHg) | |
|---|---|---|---|---|---|
| CSF/Saline | 5 | 37.1 ± 0.2 | 1.1 ± 0.8 | 310 ± 12 | 102 ± 4 |
| Muscimol/Saline | 8 | 37.5 ± 0.3 | 0.4 ± 0.1 | 339 ± 8 | 104 ± 4 |
| CSF/MDMA | 8 | 37.3 ± 0.1 | 1.2 ± 0.4 | 338 ± 10 | 103 ± 4 |
| Muscimol/MDMA | 8 | 37.6 ± 0.1 | 0.8 ± 0.3 | 342 ± 8 | 107 ± 2 |
Data are presented as mean ± SEM. No significant differences were detected for any parameter by a one-way ANOVA analysis.
Microinjecting muscimol into the DMH prevents MDMA-induced hyperthermia and hyperactivity
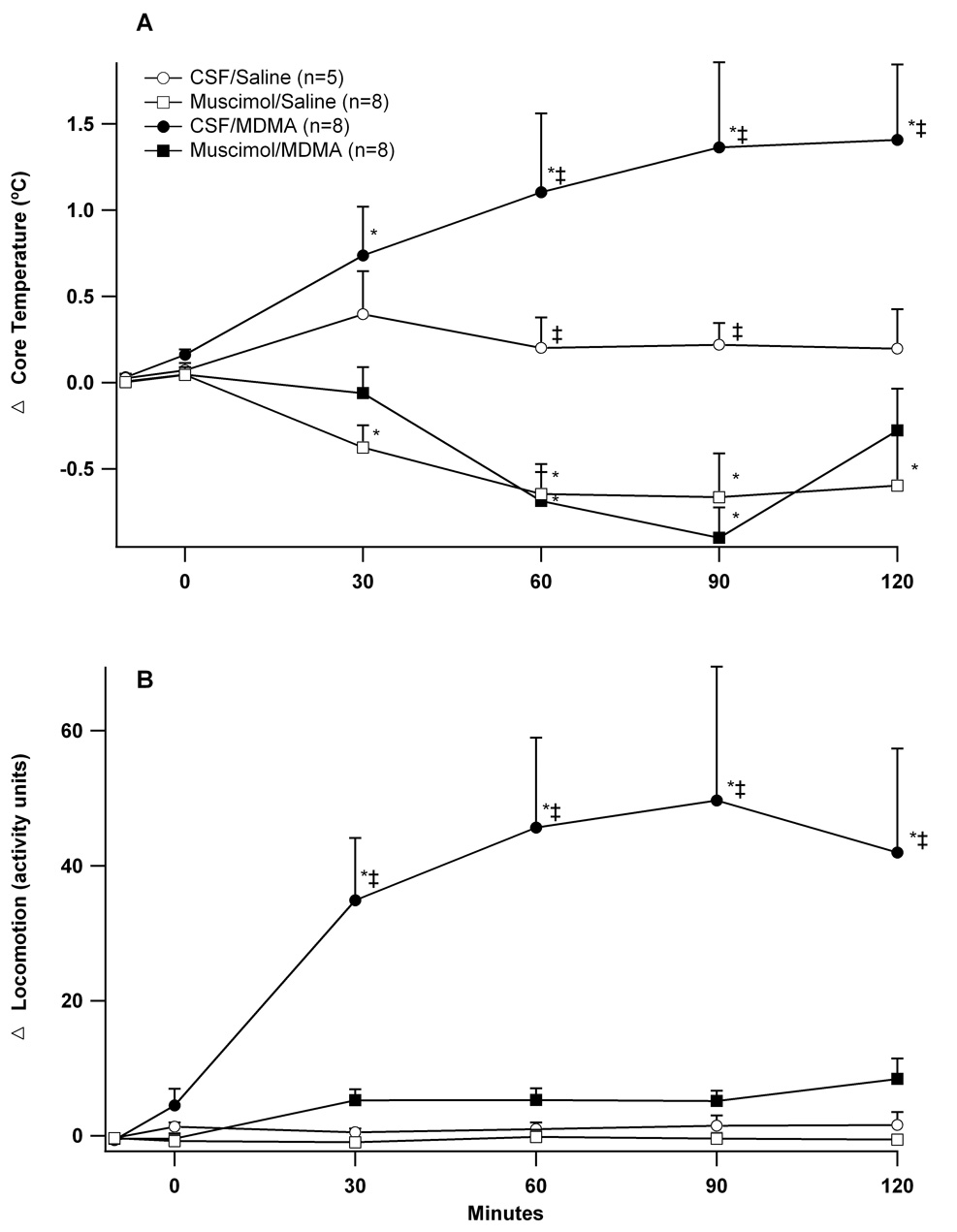
Microinjecting artificial CSF into the DMH followed by infusions of i.v. saline (CSF/Saline) had no effect on body temperature (Figure 1A). Microinjecting muscimol (80 pmol) followed by i.v. infusion of saline (Muscimol/Saline) caused a gradual decrease in body temperature reaching a nadir of 0.7 ±0.2°C below baseline at 90 minutes. Rats given MDMA after microinjections of CSF developed significant hyperthermia with core temperatures rising 1.4 ± 0.3°C above baseline by 120 minutes. Prior microinjections of muscimol completely prevented MDMA-induced hyperthermia with animals instead developing modest decreases in body temperature. These decreases were statistically identical to those seen after microinjecting muscimol followed by saline.
Figure 1. Effect of microinjection of muscimol into the DMH on temperature and locomotor activity to intravenous MDMA.
Changes from baseline over time (minutes) in (A) core temperature and (B) locomotion after microinjecting (t= −5 min) CSF (100nl) or muscimol (80 pmol/100 nl) into the DMH followed by (t = 0 min) intravenous administration of MDMA (7.5 mg/kg) or saline. *significantly different from baseline; ‡significantly different from all other groups at the specified time point. ANOVA and LSD, p < 0.05.
Microinjecting either CSF or muscimol into the DMH followed by i.v. infusion of saline did not affect activity (Figure 1B). Infusion of MDMA after microinjection of CSF increased locomotion 50 ± 6 a.u. above baseline by 90 minutes. Microinjecting muscimol into the DMH prior to i.v. infusion of MDMA abolished this effect.
Microinjecting muscimol into the DMH attenuates MDMA-induced cardiovascular responses
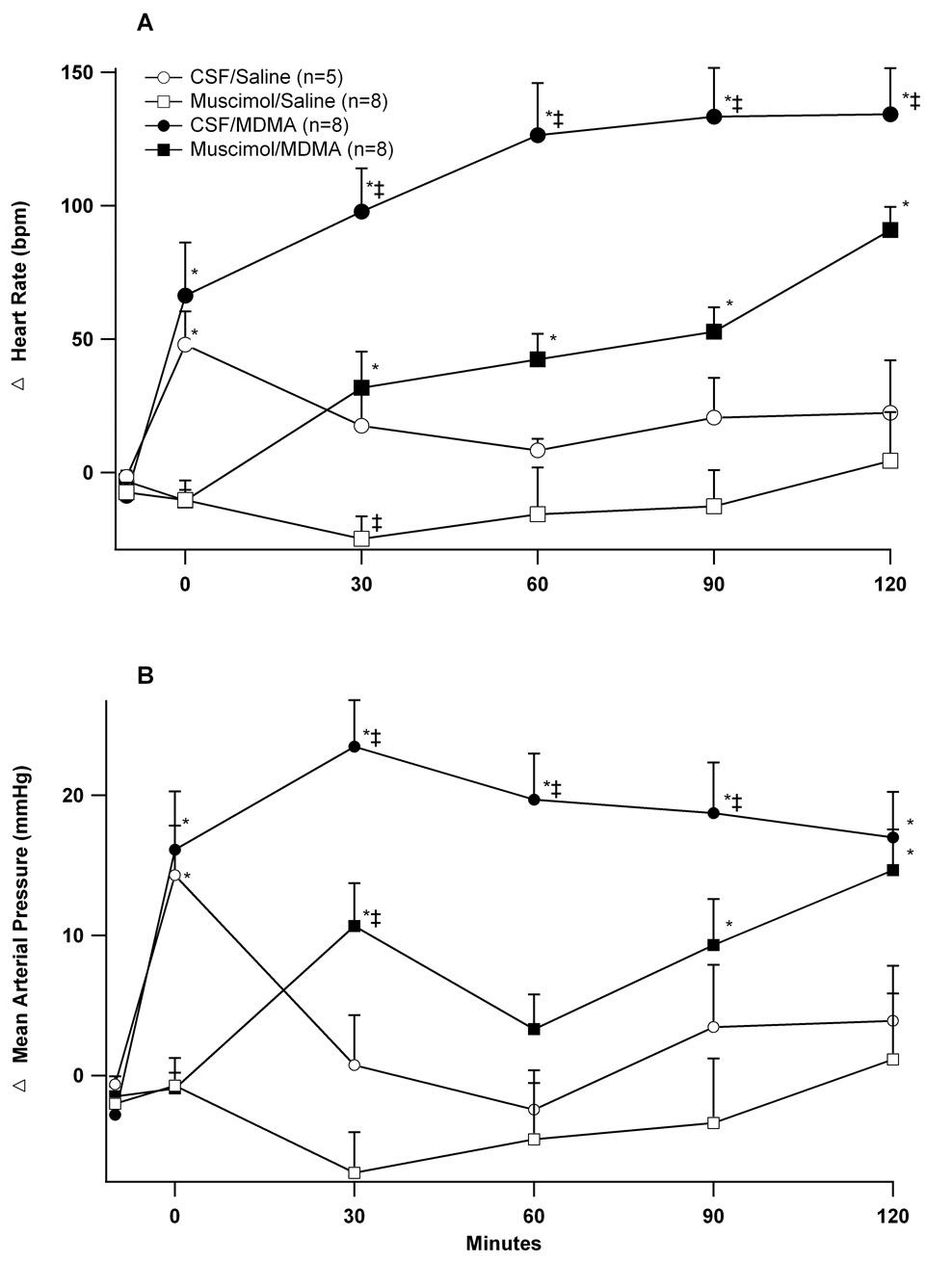
Microinjecting CSF caused transient increases in heart rate and blood pressure prior to the intravenous injections of saline or MDMA (time 0, Figures 2A and 2B). These increases were absent in animals microinjected with muscimol. In animals’ microinjected with CSF, the subsequent infusion of MDMA increased heart rate 136 ± 15 beats/min above baseline at 120 minutes and increased mean arterial pressure 24 ± 4 mmHg above baseline at 30 minutes. Microinjecting muscimol into the DMH prior to MDMA significantly attenuated these responses (Figures 2A and B).
Figure 2. Effect of microinjection of muscimol into the DMH on cardiovascular responses to intravenous MDMA.
Changes over time (minutes) in (A) heart rate and (B) mean arterial pressure after microinjecting into the DMH (t= −5 min) CSF (100nl) or muscimol (80 pmol/100 nl) followed by (t = 0 min) intravenous administration of MDMA (7.5 mg/kg) or saline. *significantly different from baseline; ‡significantly different from all other groups at the specified time point. ANOVA and LSD, p < 0.05.
4. Discussion
Results of this study demonstrate that neuronal activity in the DMH plays a role in the thermogenic, cardiovascular and behavioral responses induced by MDMA. This study represents the first time a discrete brain region has been shown to be involved in both the sympathetic and behavioral responses induced by MDMA. As MDMA’s physiologic responses, especially hyperthermia, are linked to human deaths and animal neurotoxicity (Cadet et al., 2007; Gowing et al., 2002), further characterizing the relevant neurons in the DMH and the role they play in these responses may provide insight into new treatment strategies.
Physiologic responses evoked by MDMA and stress are similar
In a response highly conserved across mammalian species, emotional stress evokes an integrated pattern of endocrine, autonomic and behavioral changes. Among these changes are increases in heart rate, mean arterial pressure, body temperature, locomotion, and circulating levels of corticosterone. These responses, as evident in Figure 1 and Figure 2, are similar to those evoked by MDMA. Similar to stress, MDMA also increases circulating levels of corticosterone (Nash et al., 1988). As previous and current findings demonstrate, the similar physiologic responses to stress and MDMA depend on neuronal activity in a common hypothalamic area – the DMH. Although abused by humans presumably for its pleasurable psychoactive effects, it is conceivable that MDMA’s effects in the laboratory animal may not be pleasurable but rather stressful. If true, this could create difficulties in separating effects directly attributable to MDMA’s pharmacologic action from effects mediated by the stress this agent creates.
We do not believe this to be a problem in the responses we studied. In anesthetized animals, a condition in which animals are incapable of experiencing exteroceptive or “emotional” stress, administration of MDMA still evokes increases in heart rate, mean arterial pressure and body temperature (Blessing et al., 2003; Rusyniak et al., 2005a; Rusyniak et al., 2005b). Likewise, in humans recreational doses of MDMA (doses chosen by users presumably for pleasure-provoking responses) cause increases in temperature, heat rate, blood pressure and cortisol similar to those observed in conscious animal models (de la Torre et al., 2000). Therefore, although the physiologic responses elicited by MDMA in the present study are similar to those evoked by stress in conscious rats, they are unlikely to be a consequence of drug-induced stress.
How DMH neurons may be involved in MDMA evoked hyperthermia
The findings of this study represent the first time a discrete brain region has been implicated in the thermogenic response to MDMA. Our choice of muscimol as a neuronal inhibitor is based on its ability, by virtue of its GABAA receptor agonist properties, to inhibit virtually all mammalian neurons. It is these properties that have made microinjections of muscimol a standard technique to achieve acute reversible suppression of neuronal activity in discrete brain regions. One inherent difficulty in microinjection studies is the possibility that effects may arise from diffusion or spread of microinjectate to regions adjacent or even relatively distant to the actual site of injection. In this study we do not believe this to be the case. Previous work from our laboratory demonstrated that microinjection of an identical dose (80 pmol) and volume (100 nl) of muscimol into the DMH inhibits both the cardiovascular and neuroendocrine responses to experimental stress (Stotz-Potter et al., 1996a; Stotz-Potter et al., 1996b). However, identical injection of muscimol at sites as little as 500 microns anterior to the region of the DMH was approximately half as effective and completely ineffective when microinjected directly into the nearby paraventricular nucleus (Stotz-Potter et al., 1996a; Stotz-Potter et al., 1996b). Thus, microinjection of muscimol in this manner has been shown to produce effects with a high degree of anatomical resolution. Furthermore, the region around our microinjection sites includes a highly compact group of neurons known to project to sympathetic premotor neurons in the raphe pallidus. When disinhibited, these neurons evoke cardiovascular responses similar to those we report in this study after a systemic dose of MDMA. induced by systemic administration of MDMA (Samuels et al., 2002). Therefore, while the involvement of adjacent regions cannot be definitively excluded, it seems most likely that the site of action of muscimol and consequently the location of neurons relevant to the observed effects of MDMA is the DMH.
The finding that inhibition of the DMH prevents MDMA-mediated hyperthermia leads to several questions: Does MDMA act directly on neurons in the DMH; are monoamines released in the DMH by MDMA; and do monoamine receptors within the DMH contribute to MDMA-induced hyperthermia? By a mechanism involving binding to monoamine transporters, MDMA increases extracellular tissue concentrations of norepinephrine, serotonin, and dopamine in various brain regions including the hippocampus, striatum, nucleus accumbens and prefrontal cortex (Gudelsky and Yamamoto, 2007; White et al., 1996). None of these sites, however, are thought to be involved in MDMA-evoked hyperthermia. Although not specifically studied, MDMA may have direct effects within the DMH. The DMH has high concentrations of both serotonin and norepinephrine transporters and to a lesser extent dopamine transporters (Hoffman et al., 1998). The DMH also has high concentrations of dopamine and norepinephrine (Moore and Bloom, 1979) and either stimulating neurons in the DMH by local infusion of the GABAA antagonist bicuculline methiodide (BMI) or exposing rats to restraint stress increases the extracellular release and tissue concentrations of serotonin, dopamine and norepinephrine (Kvetnansky et al., 1977; Lowry et al., 2003; Shekhar et al., 2002). Whether similar increases occur after MDMA and whether they result from the direct effects of MDMA in the DMH or secondary to activation of the DMH from upstream sites requires further study. Similarly, determination of the physiologic consequence of MDMA-induced increases in monoamine release in the DMH will also require further investigation.
MDMA activates the sympathetic nervous system to increase the production of heat by brown adipose tissue (Blessing et al., 2006) and to constrict cutaneous blood vessels, thus reducing dissipation of body heat (Pedersen and Blessing, 2001). Together these effects contribute to hyperthermia. Our finding that inhibiting neurons in the DMH prevents MDMA-evoked hyperthermia may reflect the role that neurons in the DMH play in controlling heat generation in interscapular brown adipose tissue (iBAT) and possibly cutaneous blood flow (Dimicco and Zaretsky, 2007). Activating neurons in the DMH increases sympathetic outflow to and heat production in iBAT (Cao et al., 2004; Zaretskaia et al., 2002). This effect is thought to be mediated by efferent connections from the DMH to the medullary raphe pallidus (RPa) where sympathetic premotor neurons controlling metabolic activity in iBAT are located (Cao et al., 2004). Similarly, via the RPa, electrically stimulating the DMH constricts vascular beds in the ear pinna of the rabbit (Nalivaiko and Blessing, 2001), the thermoregulatory counterpart to the rat tail. Therefore, inhibiting the DMH may suppress MDMA-evoked hyperthermia by preventing iBAT thermogenesis and cutaneous vasoconstriction. Further studies are needed to confirm this possibility.
Drugs that lower body temperature typically prevent MDMA-mediated hyperthermia. After the administration of these drugs, a subsequent dose of MDMA commonly causes hypothermia (Malberg et al., 1996). This makes interpretation difficult, as prevention of hyperthermia may not represent a specific pharmacologic effect, but rather the net sum of two opposing responses. In our study, modest decreases from baseline in body temperature were seen after microinjection of muscimol into the DMH in rats treated with either vehicle (from 37.5 ± 0.3 to 36.8 ± 0.2°C) or MDMA (from 37.6 ± 0.1 to 36.7 ± 0.2 °). These decreases were not only statistically identical between the groups, the decrease in the Muscimol/MDMA group is insufficient to merely oppose the hyperthermia in the CSF/MDMA group. Therefore, muscimol’s effect on preventing increases in body temperature evoked by MDMA appears to represent specific inhibition and not the result of a simple combination of opposing physiologic responses (i.e. hyperthermia by MDMA and hypothermia by microinjection of muscimol).
Microinjections of muscimol into the DMH also prevent hyperactivity induced by MDMA. Although motor activity generates heat, previous work suggests that the locomotor and hyperthermic responses induced by MDMA in conscious rats are disassociated (Dafters, 1994; Rusyniak et al., 2007). Further supporting this are studies showing that MDMA evokes-hyperthermia in animals anesthetized and unable to move (Blessing et al., 2003; Rusyniak et al., 2003; Rusyniak et al., 2005a). It follows, therefore, that the abolition of MDMA-induced hyperthermia by inhibiting the DMH is not merely a consequence of the suppression of locomotor activity.
How DMH neurons may be involved in behavioral and psychologic effects evoked by MDMA
In a dose-dependent manner, MDMA causes increases in spontaneous locomotion in rats, a response that is also seen in the serotonin behavioral syndrome (Spanos and Yamamoto, 1989). MDMA-and amphetamine-induced locomotion are classically attributed to increases in monoaminergic activity in nigrostriatal and mesocorticolimbic circuits (Bankson and Cunningham, 2001; Gold et al., 1989; Selken and Nichols, 2007). Our finding that inhibiting neurons in the DMH prevents MDMA-induced locomotion represents, to our knowledge, the first time a site outside these areas has been implicated in MDMA-induced hyperactivity. Previous work suggests that the DMH may be involved in increased locomotor activity and anxiety- and panic-related behavior. When disinhibited, neurons in the DMH evoke increased locomotor activity (Bailey and Dimicco, 2001; Shekhar and DiMicco, 1987) and when these neurons are lesioned decreases in spontaneous locomotor activity ensue (Chou et al., 2003). Along with increasing locomotion, stimulating or disinhibiting neurons in the DMH increases experimentally-induced anxiety in rats (Shekhar et al., 1990). MDMA, likewise provokes anxiety in both humans (Liechti et al., 2005) and rats (Morley and McGregor, 2000). The role that the DMH plays in anxiogenic responses to MDMA remains to be determined.
How DMH neurons may be involved in MDMA-evoked cardiovascular responses
MDMA, presumably by activation of the SNS, increases heart rate and blood pressure in animals and humans (Dumont and Verkes, 2006; Rusyniak et al., 2007). In the present study, we show that neurons responsible, at least in part, for MDMA-induced cardiovascular stimulation reside in the DMH. Previous work also demonstrates that disinhibiting neurons in the DMH by microinjection of BMI increases heart rate and blood pressure (Cao et al., 2004; DiMicco et al., 1986) and inhibiting these neurons by microinjecting muscimol prevents experimental stress-induced increases in heart rate and blood pressure (DiMicco et al., 2006; Stotz-Potter et al., 1996b). These responses involve efferent projections from the DMH to premotor neurons located in the RPa (Cao et al., 2004; Samuels et al., 2002) and the rostral ventrolateral medulla (Fontes et al., 2001). Whether cardiac stimulation elicited by MDMA involves similar pathways is not yet known.
Conclusions
Activity of neurons in the DMH appears to play a key role in both the physiologic and behavioral responses to MDMA in rats. Characterizing the mechanisms through which these neurons contribute to these effects may lead to improved treatments for acute toxicity produced by MDMA and other substituted amphetamines.
4. Procedures and Methods
All procedures conformed to guidelines set forth by NIH and were approved by the Institutional Animal Care and Use Committee. Male Sprague–Dawley rats (300 ± 10 g; Harlan, Indianapolis, IN, USA), maintained in 12-hour light:dark cycle and fed ad libitum, were anesthetized (either 50 mg/kg pentobarbital, or 80 mg/kg ketamine and 11.5 mg/kg xylazine, i.p. supplemented as needed) and a telemetric transmitter was implanted intraperitoneally (Model TA11PA-PXT50; Data Sciences, MN, USA) as described previously (Zaretsky et al., 2003). Implantation of femoral venous catheters involved the following: inserting a 20 cm length of Tygon tubing (Small Parts Inc., FL, USA) into the femoral vein; routing it subcutaneously; exteriorizing between the shoulder blades; fixing it to a harness (Kent Scientific, Torrington, CT, USA); and flushing it with saline. Rats were then placed in a stereotaxic apparatus with the incisor bar set 3.3 mm below the interaural line for placement of microinjection guide cannulae (26 gauge, Plastics One, Roanoke, VA, USA) into the right and left DMH (coordinates using a 10 degree angle from the sagittal plane: AP −3.1 mm; LR ±2.0mm; HD −7.2 mm; bregma as reference point). Once inserted, cannulae were secured using three stainless steel screws, Vetbond glue (3M, St. Paul, MN, USA) and cranioplastic cement. Afterward dummy wire cannulae were inserted in the guides, and rats were returned to their home cages for recovery. Once within 10g of their pre-surgery weights (typically 7–10 days), animals were randomized (using a random number generator) to one of four groups. Group names are defined as microinjection solution/intravenous injection: CSF/Saline (n=5); CSF/MDMA (n=8); Muscimol/Saline (n=8); or Muscimol/MDMA (n=8). Experiments were performed between the hours of 10.00 a.m. and 2.00 p.m. during the animals light phase in a laboratory with ambient temperature maintained between 24–25°C. Rats were placed in their home cage on a telemetry receiver plate and acclimated to the room for at least 2 hours. A microinjector (33 gauge, Plastics One Inc., Roanoke, VA, USA) was then connected to a 10 µl Hamilton syringe with Teflon FEP tubing (i.d. = 0.12 mm; o.d. = 0.65 mm; BASI, Lafayette, Indiana, USA) and the entire system was loaded with artificial CSF or with a solution of muscimol (80 pmol/100µl; Sigma-Aldrich, St. Louis, MO) both solutions contained 0.25% fluorescent microspheres (0.04 µm in diameter; Invitrogen, Carlsbad, California), to mark the exact site of injection. The dummy cannula was removed and the microinjector inserted into the guide cannula. A Hamilton syringe mounted in an infusion pump (KD-200, KD Scientific, Holliston, MA, USA) delivered a volume of 100 nL of injectate over 30 s, first through the right then, two minutes later, through the left cannula. After each microinjection, the injector was left in place for 90 seconds to prevent backflow upon removal. Five minutes after the last microinjection, animals were given equal volumes of either saline (0.8 ml) or MDMA (7.5 mg/kg; NIH), by slow (~120 seconds) intravenous (i.v.) infusion, and monitored for at least 120 minutes. The dose of MDMA (7.5 mg/kg) used in this study was chosen to mimic both the dose and effect of a serious human intoxication. The route (intravenous) was chosen to minimize the physiologic and behavioral effects produced by the stress of subcutaneous and intraperitoneal injections. At the conclusion of each session, rats were injected with pentobarbital (100 mg/kg, i.v.), a 14 gauge feeding tube was placed in the left ventricle and perfused with 30 ml of cold saline (4°C) followed by 30 ml of 4% paraformaldehyde. The brains were removed and postfixed in 4% paraformaldehyde overnight or longer, followed by placement in a solution saturated with 30% sucrose.
Brains were cut into 40µm coronal sections on a freezing microtome, the sections were mounted on slides, and injection sites were determined using a fluorescent microscope according to the atlas of Paxinos and Watson (1998) by an observer blinded to group allocation.
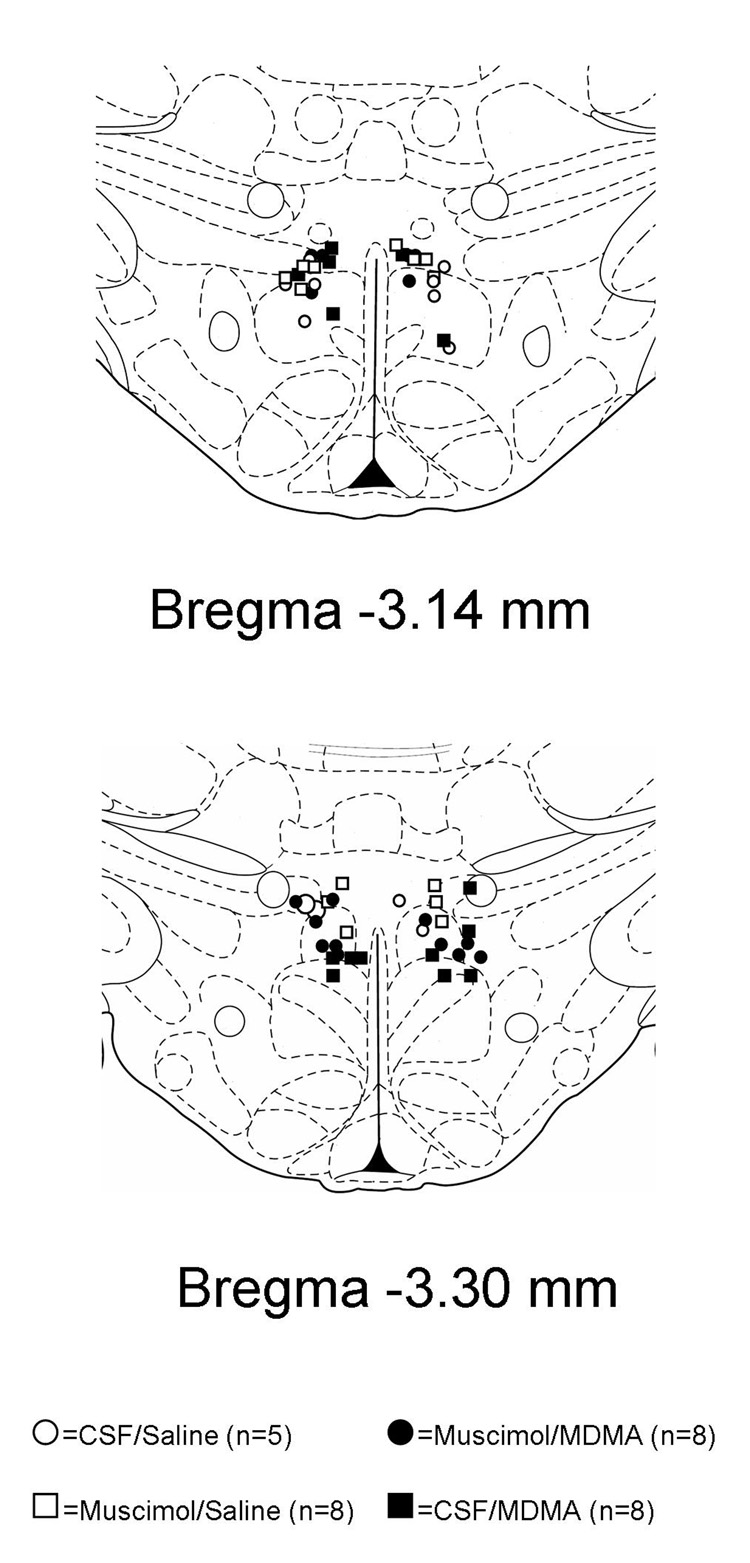
For this study, the DMH was defined as an area bounded ventrally and laterally by the inferior and lateral borders of fornix, dorsally by the mammillothalamic track, and contained anteriorly and posteriorly in the region −3.14 to −3.30 mm from bregma. Microinjection of BMI into this region has been previously shown to induce tachycardia and thermogenic responses (Samuels et al., 2004; Zaretskaia et al., 2002). All injection sites were within the DMH as defined above (Figure 3).
Figure 3. Sites of injection into the DMH.
Schematic coronal sections of the rat brain adapted from the atlas of Paxinos and Watson (1998) illustrating approximate sites of injections into the DMH. Numbers indicate distance from bregma in millimeters. Open symbols represent experiments in which animals were infused with intravenous saline after microinjections of 100 nl of CSF (open circles) or 80 pmol of muscimol (open squares) into the DMH. Filled symbols represent experiments in which animals were infused with intravenous MDMA (7.5 mg/kg) after microinjections with either 100 nl of CSF (closed circles) or 80 pmol of muscimol (closed squares). The numbers of animals in each group is given in parenthesis.
Data Analysis
Data were captured using the Dataquest A.R.T data acquisition system (Data Sciences, St. Paul,MN, USA). Of note activity, counts are arbitrary measures of activity. They are generated by changes detected in signal strength as the animal moves around. The data was statistically analyzed using SPSS 15.0 for Windows (Chicago, Illinois, USA), and graphed using Igor Pro software (WaveMetrics, Portland, OR). Grouped data are reported as change from baseline (10 minute means ± one standard error measurement) for the time points shown. We performed a two way full factorial repeated measures analysis of variance including in the model main effects for study group, time, and their interaction. We performed post hoc comparisons among time points (within a study group) and between study groups (within a time point). Pair-wise comparisons were adjusted using an LSD procedure.
Acknowledgments
This work was supported by United States Public Health Service Grants DA20484 (DER) and NS19883 (JAD).
Abbreviations
- MDMA
3,4-methylenedioxymethamphetamine
- DMH
dorsomedial hypothalamus
- BMI
bicuculline methiodide
- CSF
cerebral spinal fluid
- GABA
Gamma-aminobutyric acid
- iBAT
interscapular brown adipose tissue
- i.v.
intravenous
- MAP
mean arterial pressure
- RPa
rostral raphe pallidus
- SNS
sympathetic nervous system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of this work was presented as a poster at the 2007 Neuroscience meeting in San Diego California
References
- Aguirre N, Ballaz S, Lasheras B, Del Rio J. MDMA ('Ecstasy') enhances 5-HT1A receptor density and 8-OH-DPAT-induced hypothermia: blockade by drugs preventing 5-hydroxytryptamine depletion. European Journal of Pharmacology. 1998;346:181–188. doi: 10.1016/s0014-2999(98)00062-4. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Dimicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R8–R15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin-dopamine interactions. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- Blessing WW, Seaman B, Pedersen NP, Ootsuka Y. Clozapine reverses hyperthermia and sympathetically mediated cutaneous vasoconstriction induced by 3,4-methylenedioxymethamphetamine (ecstasy) in rabbits and rats. J Neurosci. 2003;23:6385–6391. doi: 10.1523/JNEUROSCI.23-15-06385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Zilm A, Ootsuka Y. Clozapine reverses increased brown adipose tissue thermogenesis induced by 3,4-methylenedioxymethamphetamine and by cold exposure in conscious rats. Neuroscience. 2006;141:2067–2073. doi: 10.1016/j.neuroscience.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11(1):183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Rempel N, Peng RY, Geyer MA. Serotonin 5-HT1-like receptors mediate hyperactivity in rats induced by 3,4-methylenedioxymethamphetamine. Neuropsychopharmacology. 1992;7:113–127. [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafters RI. Effect of ambient temperature on hyperthermia and hyperkinesis induced by 3,4-methylenedioxymethamphetamine (MDMA or "ecstasy") in rats. Psychopharmacology. 1994;114:505–508. doi: 10.1007/BF02249342. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Roset PN, Lopez CH, Mas M, Ortuno J, Menoyo E, Pizarro N, Segura J, Cami J. Pharmacology of MDMA in humans. Ann N Y Acad Sci. 2000;914:225–237. doi: 10.1111/j.1749-6632.2000.tb05199.x. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Abshire VM, Hankins KD, Sample RH, Wible JH., Jr Microinjection of GABA antagonists into posterior hypothalamus elevates heart rate in anesthetized rats. Neuropharmacology. 1986;25:1063–1066. doi: 10.1016/0028-3908(86)90203-0. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Stotz-Potter EH, Monroe AJ, Morin SM. Role of the dorsomedial hypothalamus in the cardiovascular response to stress. Clin Exp Pharmacol Physiol. 1996;23:171–176. doi: 10.1111/j.1440-1681.1996.tb02592.x. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacology, Biochemistry & Behavior. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Sarkar S, Zaretskaia MV, Zaretsky DV. Stress-induced cardiac stimulation and fever: Common hypothalamic origins and brainstem mechanisms. Auton Neurosci. 2006 doi: 10.1016/j.autneu.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, Verkes RJ. A review of acute effects of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20:176–187. doi: 10.1177/0269881106063271. [DOI] [PubMed] [Google Scholar]
- Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol. 2001;280:H2891–H2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- Gold LH, Geyer MA, Koob GF. Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs. NIDA Res Monogr. 1989;94:101–126. [PubMed] [Google Scholar]
- Gowing LR, Henry-Edwards SM, Irvine RJ, Ali RL. The health effects of ecstasy: a literature review. Drug Alcohol Rev. 2002;21:53–63. doi: 10.1080/09595230220119363. [DOI] [PubMed] [Google Scholar]
- Green AR, O'Shea E, Colado MI. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. European Journal of Pharmacology. 2004;500:3–13. doi: 10.1016/j.ejphar.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopamineric, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2007 doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin DV, Liu S, Ullrich T, Rice KC, Cunningham KA. Role of the serotonin 5-HT2A receptor in the hyperlocomotive and hyperthermic effects of (+)-3,4-methylenedioxymethamphetamine. Psychopharmacology (Berl) 2005;178:505–513. doi: 10.1007/s00213-004-2030-4. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Hansson SR, Mezey É, Palkovits M. Localization and Dynamic Regulation of Biogenic Amine Transporters in the Mammalian Central Nervous System. Frontiers in Neuroendocrinology. 1998;19:187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Palkovits M, Mitro A, Torda T, Mikulaj L. Catecholamines in individual hypothalamic nuclei of acutely and repeatedly stressed rats. Neuroendocrinology. 1977;23:257–267. doi: 10.1159/000122673. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Kunz I, Kupferschmidt H. Acute medical problems due to Ecstasy use. Case-series of emergency department visits. Swiss Med Wkly. 2005;135:652–657. doi: 10.4414/smw.2005.11231. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Plant A, Shanks N, Ingram CD, Lightman SL. Anatomical and functional evidence for a stress-responsive, monoamine-accumulating area in the dorsomedial hypothalamus of adult rat brain. Horm Behav. 2003;43:254–262. doi: 10.1016/s0018-506x(02)00009-0. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Sabol KE, Seiden LS. Co-administration of MDMA with drugs that protect against MDMA neurotoxicity produces different effects on body temperature in the rat. Journal of Pharmacology and Experimental Therapeutics. 1996;278:258–267. [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Morley KC, McGregor IS. (+/−)-3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") increases social interaction in rats. Eur J Pharmacol. 2000;408:41–49. doi: 10.1016/s0014-2999(00)00749-4. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Blessing WW. Raphe region mediates changes in cutaneous vascular tone elicited by stimulation of amygdala and hypothalamus in rabbits. Brain Res. 2001;891:130–137. doi: 10.1016/s0006-8993(00)03210-8. [DOI] [PubMed] [Google Scholar]
- Nash JF, Jr, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. Journal of Pharmacology & Experimental Therapeutics. 1988;245:873–879. [PubMed] [Google Scholar]
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) in conscious rabbits. Journal of Neuroscience. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyniak DE, Scruggs SL, Kamendulis LM, Klaunig JE. Ecstasy's (MDMA's) Effect on Oxidative Phosphorylation in Isolated Rat Liver Mitochondria. Acad Emerg Med. 2003;10:510. [Google Scholar]
- Rusyniak DE, Inui TS, Zaretsky DV, DiMicco JA. Society for Academic Emergency Medicine. Vol. NY, NY: 2005a. Neurons in the dorsomedial hypothalamus mediate physiologic effects of MDMA in anesthetized rats. ed.^eds. [Google Scholar]
- Rusyniak DE, Tandy SL, Hekmatyar SK, Mills E, Smith DJ, Bansal N, MacLellan D, Harper ME, Sprague JE. The role of mitochondrial uncoupling in 3,4-methylenedioxymethamphetamine-mediated skeletal muscle hyperthermia and rhabdomyolysis. Journal of Pharmacology and Experimental Therapeutics. 2005b;313:629–639. doi: 10.1124/jpet.104.079236. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. 3,4-Methylenedioxymethamphetamine- and 8-hydroxy-2-di-n-propylaminotetralin-induced hypothermia: role and location of 5-hydroxytryptamine 1A receptors. J Pharmacol Exp Ther. 2007;323:477–487. doi: 10.1124/jpet.107.126169. [DOI] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J Physiol. 2002;538:941–946. doi: 10.1113/jphysiol.2001.013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol. 2004;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- Selken J, Nichols DE. Alpha1-adrenergic receptors mediate the locomotor response to systemic administration of (+/−)-3,4-methylenedioxymethamphetamine (MDMA) in rats. Pharmacol Biochem Behav. 2007;86:622–630. doi: 10.1016/j.pbb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, DiMicco JA. Defense reaction elicited by injection of GABA antagonists and synthesis inhibitors into the posterior hypothalamus in rats. Neuropharmacology. 1987;26:407–417. doi: 10.1016/0028-3908(87)90020-7. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Hingtgen JN, DiMicco JA. GABA receptors in the posterior hypothalamus regulate experimental anxiety in rats. Brain Res. 1990;512:81–88. doi: 10.1016/0006-8993(90)91173-e. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Katner JS, Sajdyk TJ, Kohl RR. Role of norepinephrine in the dorsomedial hypothalamic panic response: an in vivo microdialysis study. Pharmacol Biochem Behav. 2002;71:493–500. doi: 10.1016/s0091-3057(01)00688-8. [DOI] [PubMed] [Google Scholar]
- Spanos LJ, Yamamoto BK. Acute and subchronic effects of methylenedioxymethamphetamine [(+/−)MDMA] on locomotion and serotonin syndrome behavior in the rat. Pharmacology, Biochemistry and Behavior. 1989;32:835–840. doi: 10.1016/0091-3057(89)90044-0. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Brutcher RE, Mills EM, Caden DC, Rusyniak DE. Attenuation of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) induced rhabdomyolysis with a1- plus β3-adrenoreceptor antagonists. British Journal of Pharmacology. 2004 doi: 10.1038/sj.bjp.0705823. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996a;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996b;16:1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, "Ecstasy") on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Research. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, DiMicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett. 2003;340:1–4. doi: 10.1016/s0304-3940(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, DiMicco JA. Stimulation and blockade of GABA(A) receptors in the raphe pallidus: effects on body temperature, heart rate, and blood pressure in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R110–R116. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]