Figure 6.
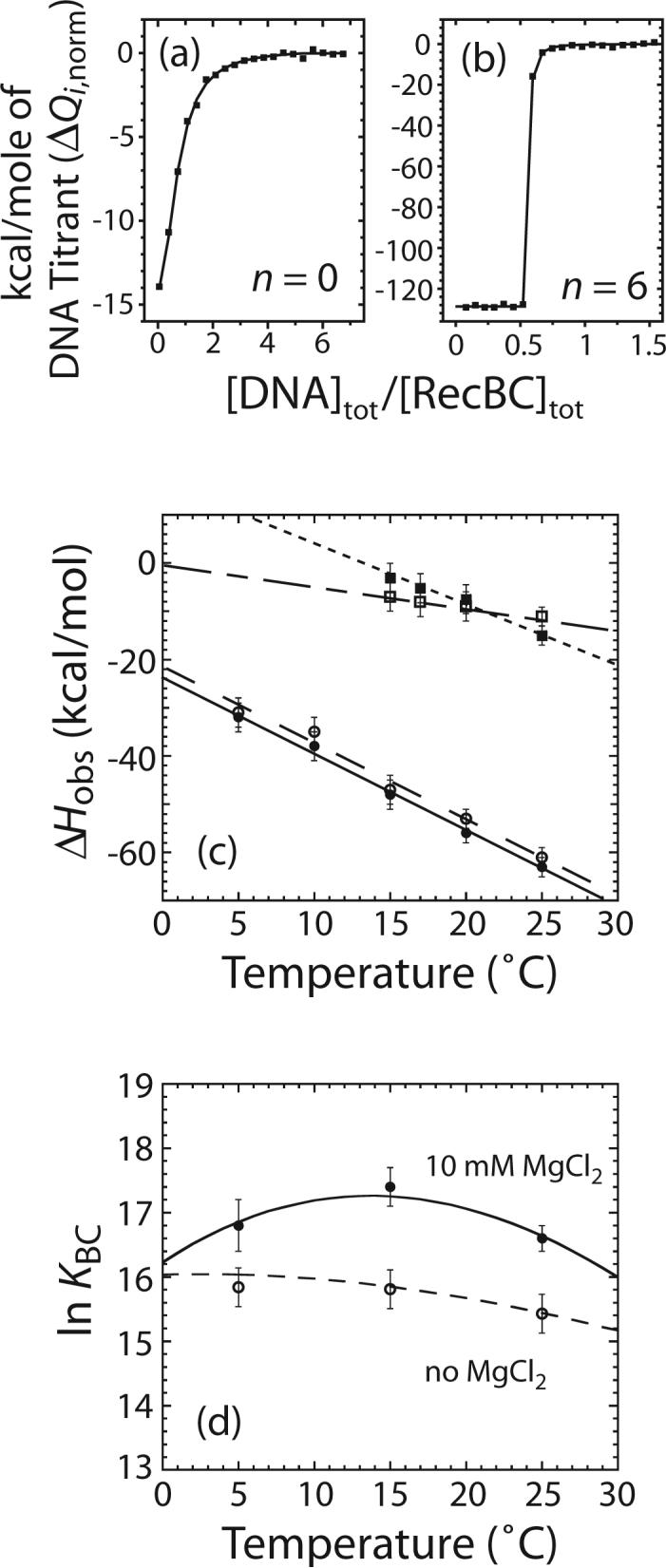
Effects of Mg2+ on the temperature dependence of the observed enthalpic change (ΔHobs) for RecBC binding to one end of the DNA VI series molecules with n = 0 or 6. Experiments were performed in buffer M plus 100 mM NaCl and the indicated [MgCl2] at the indicated temperature. (a) and (b) are representative ITC experiments to determine the enthalpic change for RecBC binding the ends of DNA VI series molecules with n = 0 or 6 in the presence of 10 mM MgCl2 at 25°C. The heat of each injection normalized to the amount of DNA injected (ΔQi,norm as defined in equation (20)) is plotted as a function of total [DNA]/total [RecBC]. (a) 710 nM RecBC was titrated with 15.2 μM DNA VI with n = 0; (b) 885 nM RecBC was titrated with 9 μM DNA VI with n = 6. Solid lines are simulations using equations (18) to (20) and the best fit values of ΔHobs (Table 2) and KBC = (1.6 ± 0.3) × 107 M−1 in (a) while K BC ≥ 109 M−1 in (b). (c) Effects of Mg2+ on values of ΔCp,obs for RecBC binding to a DNA end. ΔHobs for RecBC binding to an end of DNA VI with n = 6 in the presence of 10 mM MgCl2 (●) or in the absence of MgCl2 (○) and ΔHobs for RecBC binding to a blunt DNA end in the presence of 10 mM MgCl2 (■) or in the absence of MgCl2 (□) are plotted as a function of temperature (°C). Straight lines represent results obtained from linear least-square analysis of each set of data and the value of ΔCp,obs obtained from each set of data is presented in Table 2. (d) Effects of Mg2+ on the temperature dependence of equilibrium constant (KBC) for RecBC binding to a blunt DNA end measured by competition fluorescence titration experiments. Experiments were performed in buffer M, 100 mM NaCl with or without 10 mM MgCl2 at the indicated temperature. Values of ln KBC measured in 10 mM MgCl2 (●) or 0 mM MgCl2 (○) are plotted as a function of temperature (°C). The solid and broken lines are simulations using equation (22) and the ΔHobs and ΔCp,obs values obtained from ITC experiments in the presence or absence of 10 mM MgCl2, respectively (Table 2).
