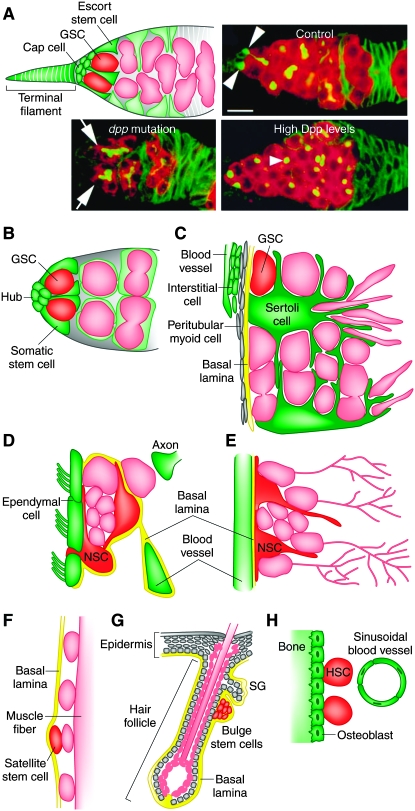
Figure 1.—
Stem cell niches. (A) Drosophila germarium illustrating GSCs in their niche, formed by cap cells, terminal filament cells, and escort stem cells (top left). Escort stem cell and GSC progeny are shown in light green and pink, respectively. Confocal images (top right and bottom) showing germaria from control, decapentaplegic (dpp) mutant, and dpp-overexpressing females. dpp encodes a BMP signal. GSCs (arrowheads) are lost in dpp mutants. Arrows point to differentiating germ-cell cysts. Increased numbers of GSC-like cells (arrowhead) result from high Dpp levels. Bar, 10 μm. Confocal images (top right and bottom) were reproduced from Figure 1D and Figure 4, A and G, in Xie and Spradling (1998). (B) Male Drosophila GSCs in their niche, comprising the hub and somatic stem cells. (C) Seminiferous epithelium in the mammalian testis. GSCs and their progeny (pink) are closely associated with Sertoli cells, and GSCs reside in proximity to the vasculature and interstitial cells. (D) The subventricular zone showing astrocytes that function both as NSCs and as niche components. NSCs are closely associated with ependymal cells, blood vessels, a specialized basal lamina, and axon terminals. (E) The subgranular zone depicting NSCs in close association with blood vessels. In D and E, NSC progeny are shown in pink. (F) Satellite stem cell (red) in the mammalian muscle. Satellite stem cells and committed satellite cells (pink ovals) reside sandwiched between the muscle fiber and the basal lamina. The depicted satellite stem cell has recently divided to produce one stem cell and one committed daughter. (G) Mammalian hair follicle and part of epidermis. Hair follicle stem cells reside in the bulge (bulge stem cells), and separate populations of stem cells reside in the basal layer of the epidermis and in the sebaceous gland (SG). (H) HSC in the bone marrow. HSCs reside in close proximity to the inner bone surface and to specialized blood vessels.