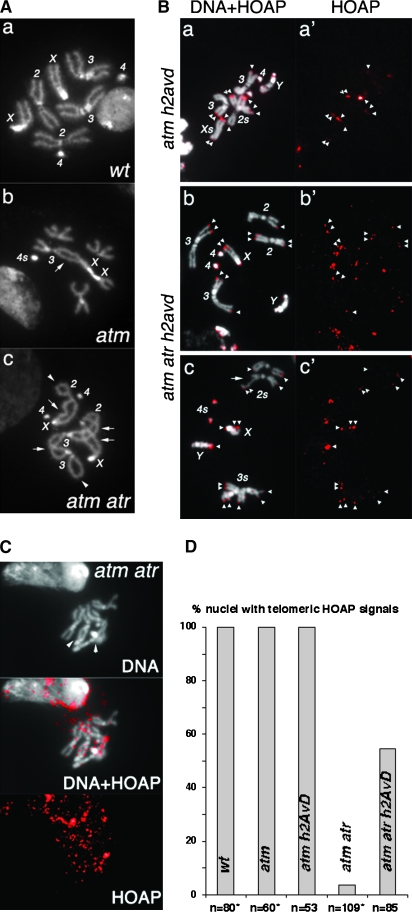
Figure 4.—
Loss of H2A.Z restores HOAP loading to telomeres defective for both ATM and ATR checkpoint functions. (A) DAPI-stained mitotic nuclei from third instar larval neuroblasts. (a) A wild-type (wt) female nucleus with all of the chromosomes labeled. (b) An atm nucleus. A double fusion between chromosomes 3 and X (arrow) is counted as two fusions in Figure 1. The two chromosome 4's are fused, which is counted as one fusion for Figure 1 because one cannot unequivocally define it as either a single or a double fusion. The short arms of two chromosome X's are also fused and counted as one fusion for the same reason. In total, four fusions were identified in this nucleus. (c) An atm atr nucleus. Two single fusions between sisters are indicated by arrowheads. Four double fusions (X–2, X–2, 2–3, 3–3) are indicated by arrows. In total, ten fusions were identified in this nucleus. (B) (a–c) Merged images of DAPI-stained mitotic chromosomes and anti-HOAP signals in red. (a′–c′) Images showing only anti-HOAP signals. Genotypes are indicated at the left. Telomeric HOAP signals are indicated by arrowheads, except those on chromosome 4's. The nucleus in a and a′ had an attached X chromosome so that the X chromosomes are about twice as long as the major autosome arms. The nucleus in c and c′ had a chromatid break (arrow) on one chromosome 2. (C) Lack of HOAP signals on atm atr mutant telomeres. The HOAP signals are overexposed. In the DNA (top), two potential fusion junctions are indicated with arrowheads. (D) The percentage of nuclei with the indicated genotypes that had HOAP signals on at least one telomere. *Data partly from Bi et al. (2004, 2005a).