Figure 4.
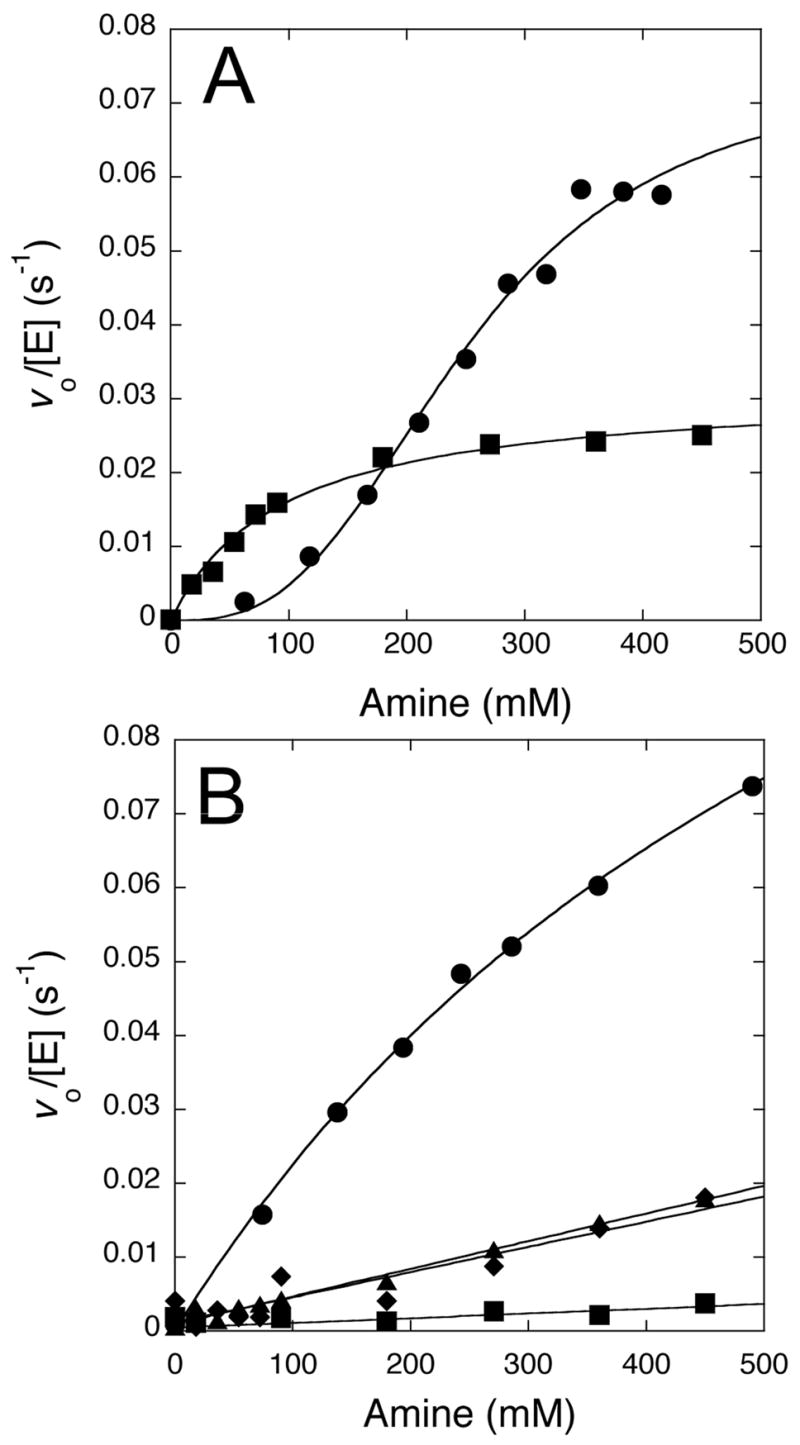
Concentration dependence of the chemical rescue reactions. A) At pH 7.3, rates of substrate disappearance increase with higher concentrations of imidazole (■, pKa 7) and hydroxylamine (●, pKa 6). Imidazole shows evidence of saturation kinetics and hydroxylamine shows sigmoidal kinetics, possibly indicative of positive cooperativity (see Results). B) At pH 9.5, rates of substrate disappearance increase with higher concentrations of methylamine (●, pKa 10.6), dimethylamine (◆, pKa 10.6), t-butylamine (▲, pKa 10.6) and 2-amino-2-methyl-1,3-propanediol (■, pKa 8.8). Methylamine shows some curvature, possibly indicative of saturation, but the remaining amines are fit by a linear concentration dependence. All experiments (except for imidazole rescue) are corrected for background rates observed in the absence of enzyme. See Experimental Details and Results for more information.
