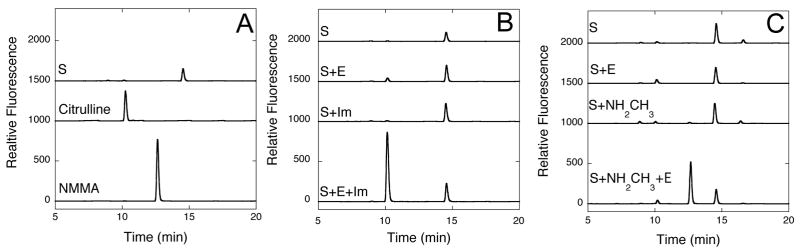
Figure 5.
HPLC of Chemical Rescue Reaction Products. A) Commercial standards of S-methyl-L-thiocitrulline (S, 14.5 min), L-citrulline (10.3 min) and Nω-methyl-L-arginine (NMMA, 12.6 min) are derivatized using o-phthaldehyde, separated on a C18 analytical column and detected using fluorescence (Ex = 338 nm; Em = 445 nm). B) Reaction products of incubations (4 h) containing S, S and H162G DDAH (E), S and imidazole (Im) and S, E and Im are derivatized and separated into their components by HPLC. In the last incubation mixture, a significant peak appearing at 10.1 min is observed, consistent with citrulline production. C) Reaction products of incubations (4 h) containing S, S and E, S and methylamine (NH2CH3), and S, NH2CH3 and E are derivatized and separated into their components. In this last incubation mixture, a significant peak appearing at 12.7 min is observed, consistent with Nω-methyl-L-arginine formation. In each plot, stacked elution traces are offset by 1000, 1500 or 2000 relative fluorescence units for easier visualization.