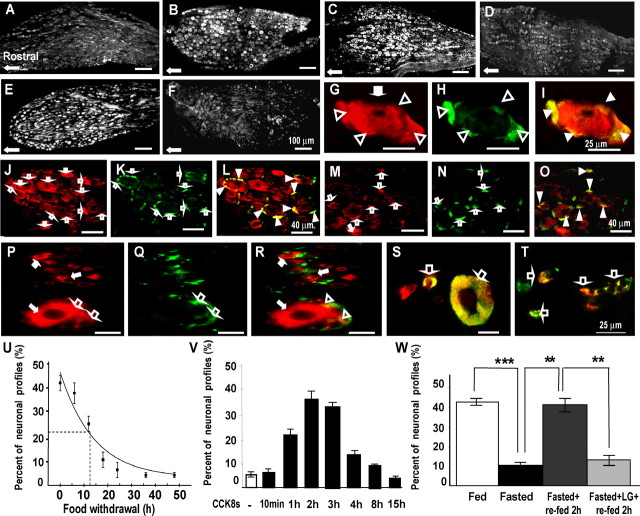
Figure 2.
CCK regulates expression of Y2R in vagal afferent neurons. A, B, Expression of Y2R in rats fasted for 48 h (A) is decreased in the mid-caudal regions of the nodose ganglion compared with rats fed ad libitum (B), although some neurons still express Y2R in fasted rats. C, After administration of CCK8s (10 nmol, i.p.) to fasted rats, there is a substantial increase of Y2R-positive neurons. D, For comparison, control samples show low abundance of Y2R 2 h after administration of vehicle. E, Refeeding of fasted rats for 2 h increases expression of Y2R in nodose neurons. F, In contrast, in rats treated with a CCK1R antagonist (lorglumide) 15 min before refeeding, the increase of Y2R after refeeding was inhibited. G–I, At higher power, dual staining with antibodies to Y2R (G, red) and glutamine synthetase (H, green) reveals that Y2R immunoreactivity is localized to satellite cells (open arrowheads) as well as neurons (filled arrows); I, overlay of panels G and H showing colocalization in satellite cells (yellow, filled arrowheads). J–L, In rats fed ad libitum, there is Y2R in both neurons (J, red, filled arrows) and satellite cells (K, green, open arrows; L, overlay with colocalization in satellite cells indicated by filled arrowheads). However, in fasted rats (M–O), there is decreased expression of Y2R in neuronal cell bodies (M, filled arrows) but not satellite cells (N, open arrows; O, overlay with colocalization in satellite cells indicated by filled arrowheads). P–T, Localization of Y2R in human nodose ganglia. P–R, Dual staining with antibodies to Y2R (P, red) and GFAP (Q, green) shows that Y2R is expressed in small and large neurons (red, filled arrows) as well as satellite cells (open arrows) (R, overlay with colocalization to satellite cells indicated by open arrowheads). S, Colocalization of Y2R (red) and CCK1R (green) in small and large neurons indicated by open arrows. T, Colocalization of Y2R (red) and VR1 (green) in small neurons (open arrows). U–W, Quantification of CCK-dependent changes in Y2R-positive neurons. U, Progressive decrease in the numbers of Y2R-immunoreactive cells in nodose ganglia after nutrient withdrawal. Exponential curve-fitting (OriginPro7.5) indicated 50% decrease in abundance of Y2R-immunoreactive neurons at 12.5 h (n = 4). V, Intraperitoneal administration of CCK8s (10 nmol) to rats fasted for 24 h increased the number of Y2R-immunoreactive neurons with a peak at 2 h (n = 4). W, Fasting for 24 h decreased the number of Y2R-immunoreactive neurons (***p < 0.001). Refeeding for 2 h increased the number of Y2R-immunoreactive neurons (**p < 0.01) and the CCK1R antagonist lorglumide administered 15 min before refeeding prevented the increase in Y2R-immunoreactive neurons (**p < 0.01) (n = 5–6). Scale bars: 100 μm in A–F, 25 μm in G–I and P–T, and 40 μm in J–O.