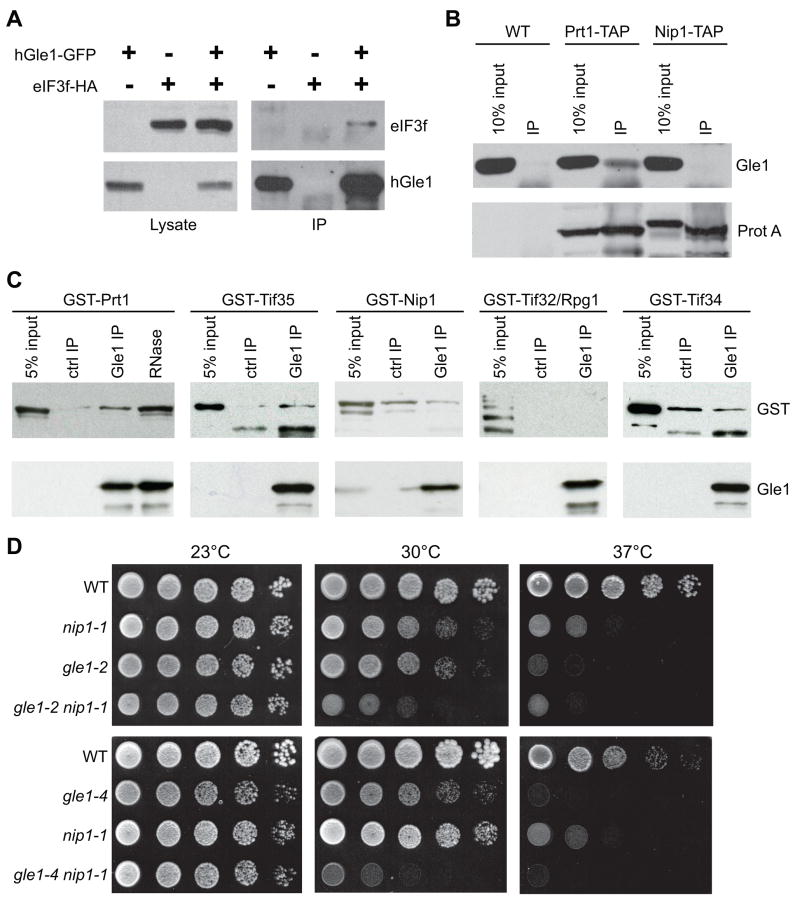
Figure 4. Gle1 has conserved interactions with eIF3 subunits.
(A) Plasmids expressing hGle1-GFP and eIF3f-HA were cotransfected into HeLa cells, and immunoprecipitations were performed using α-GFP antibodies. Lysates (5%) and immunoprecipitates were immunoblotted using α-GFP or α-HA.
(B) Immunoprecipitations were performed with lysates from PRT1-TAP, NIP1-TAP, and wild-type (WT) yeast strains. Lysates (10% of input) and total immunoprecipitates (IP) were immunoblotted with α-mouse IgG (for TAP-tagged proteins (ProtA)) or α-Gle1.
(C) Recombinant purified Gle1 was incubated with bacterial lysates for GST-Prt1, -Nip1, -Rpg1/Tif32, -Tif34, or -Tif35, and bound proteins were isolated by immunoprecipitation with α-Gle1. In controls, lysates were incubated without Gle1. RNase A treatment was conducted with the Gle1/GST-Prt1 sample. Total bound and input (5%) were immunoblotted with α-GST or α-Gle1.
(D) Cultures of the respective gle1 and nip1 single and double mutant strains with wild-type controls (WT) were serially diluted and spotted on YPD. Plates were incubated 3 days at 23°C, 30°C or 37°C.