Abstract
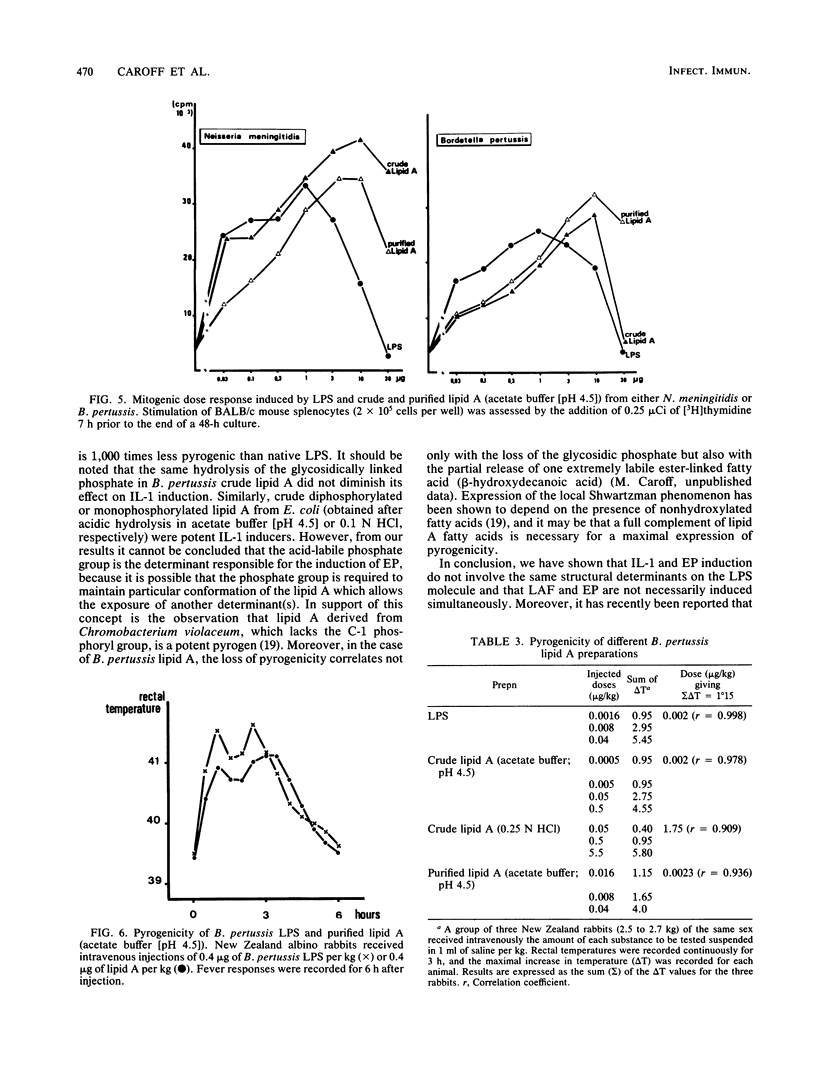
Free lipid A of Bordetella pertussis, Neisseria meningitidis, and Escherichia coli lipopolysaccharide (LPS) was prepared by hydrolysis in acetate buffer (pH 4.5); in addition, lipid A from B. pertussis and E. coli was prepared by hydrolysis in mineral acid (HCl). The precipitates obtained were purified by extraction methods in toluene-methanol and are referred to as crude lipid A. Purified lipid A from N. meningitidis and B. pertussis was obtained by extraction in a mixture of chloroform-methanol-water-triethylamine. The different preparations were tested for their pyrogenicity (endogenous pyrogen; EP) and their capacity to trigger the release of interleukin-1 (IL-1; previously known as lymphocyte-activating factor; LAF) by human monocytes. Crude lipid A from E. coli and N. meningitidis were both IL-1 inducers. Crude B. pertussis lipid A (acetate buffer; pH 4.5), which contains a beta-1-6-linked D-glucosamine disaccharide, two phosphoryl groups, and five fatty acids, was pyrogenic and an IL-1 inducer (EP+/LAF+); but crude B. pertussis lipid A (0.25 N HCl), which lacked the glycosidic phosphoryl group, was 1,000-fold less pyrogenic than the diphosphorylated lipid A, yet it retained its IL-1-inducing capacity (EP-/LAF+). Purified N. meningitidis lipid A was not an inducer of IL-1 release and purified B. pertussis lipid A exhibited identical pyrogenicity as the parent LPS but was devoid of any IL-1-release inducing capacity (EP+/LAF-). These results demonstrate that for some endotoxins, purified lipid A is unable to induce IL-1 release by human monocytes; however, it is pyrogenic, supporting the hypothesis that IL-1 and EP are induced by different determinants on the LPS molecule.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Ribi E., Cantrell J. L. Different structural requirements of endotoxic glycolipid for tumor regression and endotoxic activity. Biochem Biophys Res Commun. 1982 Jun 15;106(3):677–682. doi: 10.1016/0006-291x(82)91764-8. [DOI] [PubMed] [Google Scholar]
- Ayme G., Caroff M., Chaby R., Haeffner-Cavaillon N., Le Dur A., Moreau M., Muset M., Mynard M. C., Roumiantzeff M., Schulz D. Biological activities of fragments derived from Bordetella pertussis endotoxin: isolation of a nontoxic, Shwartzman-negative lipid A possessing high adjuvant properties. Infect Immun. 1980 Mar;27(3):739–745. doi: 10.1128/iai.27.3.739-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade H., Rietschel E. T. Alpha-2----4-interlinked 3-deoxy-D-manno-octulosonic acid disaccharide. A common constituent of enterobacterial lipopolysaccharides. Eur J Biochem. 1984 Dec 3;145(2):231–236. doi: 10.1111/j.1432-1033.1984.tb08543.x. [DOI] [PubMed] [Google Scholar]
- Caroff M., Szabó L. Identification of 2-amino-6-O-(2-amino-2-deoxy-beta-D-glucopyranosyl)-2-deoxy-D-glucose as a major constituent of the hydrophobic region of the Bordetella pertussis endotoxin. Carbohydr Res. 1983 Mar 16;114(1):95–102. doi: 10.1016/0008-6215(83)88176-2. [DOI] [PubMed] [Google Scholar]
- Cavaillon J. M., Alouf J. E. Mitogenicity of streptococcal extracellular products and antagonism with concanavalin A. Immunol Lett. 1982;5(6):317–322. doi: 10.1016/0165-2478(82)90120-1. [DOI] [PubMed] [Google Scholar]
- Cavaillon J. M., Haeffner-Cavaillon N. Polymyxin-B inhibition of LPS-induced interleukin-1 secretion by human monocytes is dependent upon the LPS origin. Mol Immunol. 1986 Sep;23(9):965–969. doi: 10.1016/0161-5890(86)90127-6. [DOI] [PubMed] [Google Scholar]
- Charon D., Chaby R., Malinvaud A., Mondange M., Szabó L. Chemical synthesis and immunological activities of glycolipids structurally related to lipid A. Biochemistry. 1985 May 21;24(11):2736–2742. doi: 10.1021/bi00332a021. [DOI] [PubMed] [Google Scholar]
- Damais C., Riveau G., Parant M., Gerota J., Chedid L. Production of lymphocyte activating factor in the absence of endogenous pyrogen by rabbit or human leukocytes stimulated by a muramyl dipeptide derivative. Int J Immunopharmacol. 1982;4(5):451–462. doi: 10.1016/0192-0561(82)90020-0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Bernheim H. A., Duff G. W., Le H. V., Nagabhushan T. L., Hamilton N. C., Coceani F. Mechanisms of fever induced by recombinant human interferon. J Clin Invest. 1984 Sep;74(3):906–913. doi: 10.1172/JCI111508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M., Moreau M., Szabó L. Interleukin 1 secretion by human monocytes stimulated by the isolated polysaccharide region of the Bordetella pertussis endotoxin. Mol Immunol. 1984 May;21(5):389–395. doi: 10.1016/0161-5890(84)90036-1. [DOI] [PubMed] [Google Scholar]
- Hanson D. F., Murphy P. A. Demonstration of interleukin 1 activity in apparently homogeneous specimens of the pI 5 form of rabbit endogenous pyrogen. Infect Immun. 1984 Aug;45(2):483–490. doi: 10.1128/iai.45.2.483-490.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Harada K., Mori Y., Kawasaki A., Tanaka A., Nagao S., Tanaka S. Immunobiologically active lipid A analogs synthesized according to a revised structural model of natural lipid A. Infect Immun. 1984 Jul;45(1):293–296. doi: 10.1128/iai.45.1.293-296.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebbar S., Cavaillon J. M., Caroff M., Ledur A., Brade H., Sarfati R., Haeffner-Cavaillon N. Molecular requirement for interleukin 1 induction by lipopolysaccharide-stimulated human monocytes: involvement of the heptosyl-2-keto-3-deoxyoctulosonate region. Eur J Immunol. 1986 Jan;16(1):87–91. doi: 10.1002/eji.1830160117. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A. Ability of human leukocytic pyrogen to enhance phytohemagglutinin induced murine thymocyte proliferation. Cell Immunol. 1981 Sep 1;63(1):134–142. doi: 10.1016/0008-8749(81)90034-4. [DOI] [PubMed] [Google Scholar]