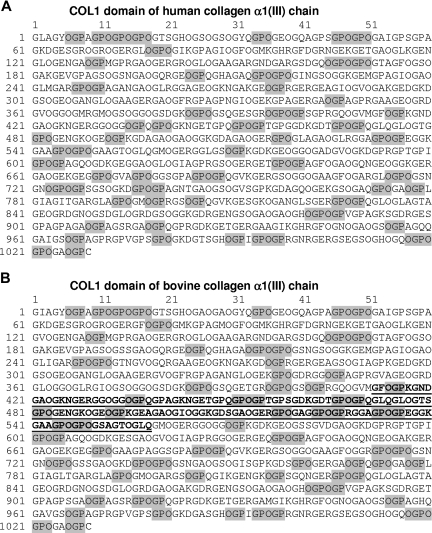
Figure 1.
Amino acid sequence of triple-helical (COL1) domain of human and bovine type III collagen. (A) Human COL1 (triple-helical) domain from collagen α1(III) chain. OGP/GPO motifs are highlighted and comprise 20% of the full sequence. The sequence is taken from Swiss-Prot P02461 (European Bioinformatics Institute, Hinxton, United Kingdom). This is the sequence used to design type III Toolkit peptides. (B) Bovine COL1 domain from collagen α1(III) chain. OGP/GPO motifs are highlighted and comprise 21% of the full sequence. The sequence of the α1(III)CB4 fragment is underlined. The sequence is taken from Swiss-Prot Q08E14. The prolines in the X′ position are shown as the posttranslational modification hydroxyproline for both the human and bovine sequences.