Abstract
The honeybee (Apis mellifera) waggle dance is one of the most intriguing animal communication signals. A dancing bee communicates the location of a profitable food source and its odour. Followers may often experience situations in which dancers indicate an unfamiliar location but carry the scent of a flower species the followers experienced previously at different locations. Food scents often reactivate bees to resume food collection at previously visited food patches. This double function of the dance creates a conflict between the social vector information and the private navigational information. We investigated which kind of information followers with field experience use in this situation and found that followers usually ignored the spatial information encoded by the waggle dance even if they followed a dance thoroughly (five waggle runs or more). They relied on private information about food source locations instead (in 93% of all cases). Furthermore, foragers preferred to follow dancers carrying food odours they knew from previous field trips, independently of the spatial information encoded in the dance. Surprisingly, neither odour identity nor the location indicated by the dancer was an important factor for the reactivation success of a dance. Our results contrast with the assumption that (i) followers usually try to decode the vector information and (ii) dances indicating an unfamiliar location are of little interest to experienced foragers.
Keywords: waggle dance, honeybee, communication, social information, private information
1. Introduction
The waggle dance of the honeybee (Apis mellifera) is probably the best-known communication signal in the insect world. A dancing bee provides its followers with at least three types of information that are important for the organization of collective foraging in honeybees: (i) the distance and direction to the exploited food source (vector information, the ‘dance language’; von Frisch 1967; Riley et al. 2005), (ii) the odour of the food source (von Frisch 1967; Wenner & Wells 1990; Diaz et al. 2007), and (iii) the presence of a profitable food source (von Frisch 1923; Wells & Wenner 1973; Thom et al. 2007). While the vector information is unique to the waggle dance, the other two types of information are involved in recruitment of many other social insects (Lindauer & Kerr 1960; Dornhaus & Chittka 1999; Hrncir et al. 2007).
Most of the bees interacting with dancers are foragers with field experience and many of them follow dances after being temporally inactive (Biesmeijer & Seeley 2005). These followers can use the vector information in order to find the location of the food source (von Frisch 1967; Riley et al. 2005). In addition, they can learn floral cues, such as the odour of the flower species, which are carried on the body and in the collected food itself (von Frisch 1967; Farina et al. 2005; Grüter et al. 2006). Both kinds of information help foragers to locate the indicated food source (von Frisch 1967).
On the other hand, temporarily inactive foragers, which possess self-acquired (private) information about the location of food sources from previous field trips, can be reactivated to resume foraging at known food sources (e.g. after nightfall, bad weather or the end of nectar or pollen production periods of particular plant species) by encountering the scent of a previously visited food source in the hive (via following round dances (von Frisch 1923) or simply by encountering the scent (Ribbands 1954; Wenner & Johnson 1966)). In such a case, the familiar scent triggers navigational and visual memories (Reinhard et al. 2004). Hence, dances provide followers with both social information for the discovery of a food source and the social context for the activation of private navigational information about a previously profitable food source.
It is largely unknown which kind of information foragers with field experience use after following waggle dances in natural situations. In other words, which strategy do followers choose when the dancer carries the odour of a familiar flower species, but indicates an unknown location? In such a situation, the waggle dance creates a conflict and a bee could either (i) use her self-acquired information and fly to memorized food source locations or (ii) use the social vector information and fly to the place indicated by the dancer.
There is preliminary support for both the strategies. On one hand, von Frisch repeatedly reported that dances with ‘mismatched’ vector information are of little interest to experienced foragers (von Frisch & Rösch 1925; von Frisch 1946, 1967). He suggested that these dances have very low reactivation success and that consequently, the vector information is the primary source of information used by experienced foragers (von Frisch 1967). Since then it has often been assumed that the vector information provided by the dance is used to discover the indicated food patch when bees follow dances (Seeley 1983; Seeley & Visscher 1988). On the other hand, Johnson (1967) observed experienced foragers following waggle dances indicating an unknown location but carrying a known odour and he reported that these foragers subsequently used the private navigational information to fly to the food location where this odour had been learnt. He concluded that bees with field experience normally ignore the vector information and rely on their olfactory memories.
Until now, experiments with quantitative and qualitative analyses of different kinds of in-hive interactions like dance following or trophallactic contacts are lacking and it is, therefore, still unclear what kind of strategy foragers pursue. Resolving this contradiction is obviously important to understand how honeybees use the waggle dance and how this signal affects collective foraging patterns at the colony level. In this experiment, we exposed inactive foragers to dancers, which indicate an unknown location but carry an odour that the inactive foragers had previously learnt at a different location. We compared this situation with an alternative one in which there is no conflict between private and social information. We analysed in-hive interactions between active foragers and inactive experienced foragers in order to quantify the attractiveness and the reactivation success of the different dance types.
2. Material and methods
Four colonies with approximately 3000 honeybees each, housed in two-frame observation hives (H1–H4) were used. Two Apis mellifera ligustica colonies (H1 and H2) were held at the experimental field of the University of Buenos Aires and two Buckfast colonies (H3 and H4; a cross between A. m. ligustica and A. mellifera mellifera) were held at the ethological field station of the University of Bern. Colonies had a queen, brood and reserves.
(a) Experimental procedure
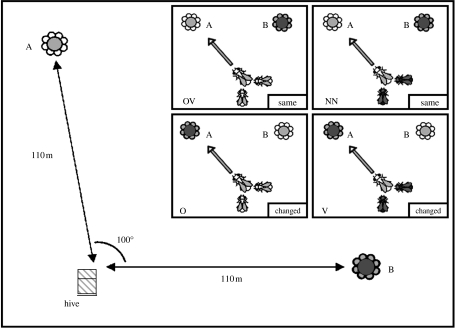
The experiment was done in 2006 (H1) and 2007 (H2–H4). We used one hive at a time to perform the experiment. Two groups of 15–30 bees coming from one hive were trained to collect a unscented 2 M sucrose solution at two different feeders with unrestricted flow 110 m from the hive. This feeder–hive distance guaranteed that our foragers showed waggle dances from which the observer could easily see which of the two feeders was advertised. The distance between the two feeders was 170 m, and the angle between the two directions from the hive to the feeders was 100° (figure 1). The bees trained to the feeders were numbered with plastic tags on the thorax (Opalithplättchen) for individual identification. One day after the two groups of foragers had been established, both feeders offered 2 M differently scented sucrose solutions (50 μl essential oil per litre sucrose solution) for 60 min from 10.00 to 11.00 in the morning (henceforth, training session). We used rose, vanilla (H1), jasmine, peppermint (H2), eucalyptus, anise (H3), lavender and lemon (H4) as scents for the two different feeders. The numbers of all foragers that collected scented food during this time were recorded. During these 60 min, foragers of both groups learnt the link between the location and the scent. Then the feeders were removed, cleaned (with water) and placed in their original position 110 m from the hive. From 11.00 to approximately 15.00 treated foragers could freely inspect the now empty feeders. From 15.00 to 15.40, we again offered 2 M scented sucrose solutions. During the 20 min immediately before offering food again, one or two foragers that inspected the feeder were captured and released at the beginning of the feeding period to start recruitment. During the feeding period, two numbered foragers per feeder were allowed to collect food and recruit other bees (henceforth, testing session). The high concentration of the offered sucrose solution guaranteed a high probability of dancing by the collecting foragers. All other foragers arriving at the two feeders after the start of this reactivation period were captured with plastic tubes after they started drinking. Usually, foragers started drinking immediately after arrival, irrespective of whether they knew the odour or not. The time of arrival and their numbers were noted. At the same time, the hive interactions between the four employed foragers (two per feeder) and the other numbered bees were filmed using a Sony DCR-TRV520 video camera. Training of bees in the morning and reactivation in the afternoon was considered one trial.
Figure 1.
Spatial experimental arrangement and behavioural categories. Arrangement of the feeding locations and the observation hive in both experimental fields in Argentina and Switzerland. A and B represent locations while dark and bright flower dummies represent different odours. The four insert figures represent the four different kinds of dance experienced by inactive forages in the ‘same-situation’ and the ‘changed-situation’. OV, experienced foragers (small bees) follow dancers (big bees) that collect the familiar odour (both dancer and follower are bright) and indicate the location of the known feeder (bright flower). NN, experienced foragers follow dancers that collect the unfamiliar odour (bright dancer versus dark followers) and indicate the location of the unknown feeder (‘same-situation’). O, experienced foragers follow dancers that collect the familiar odour, but indicate the location of the unknown feeder. V, experienced foragers follow dancers that collect the unfamiliar odour but indicate the known feeder.
We did two trials with each colony, a ‘same-situation’ trial and a ‘changed-situation’ trial. In the same-situation trial, employed foragers showed dances that created a situation of matching private and social location information. A feeder offered the same scent in the solution during the testing session as during the training session. In the changed-situation trial, we exchanged the two scents for the testing session, that is, during the testing session feeder A offered the scent that was offered by feeder B during the training session and vice versa.
Hence, dance followers with olfactory experience established at a particular feeder could experience four types of dance (figure 1; table 1). In the cases of dances providing matching olfactory information (O-dances; see table 1 for explanations) and dances providing both novel vector and olfactory information (NN-dances), a mismatch between private location and social location information occurs. In the case of NN-dances, however, the odour had not been experienced at the known feeder location. Every bee was used only once.
Table 1.
The four different types of dances of unemployed experienced foragers could encounter and the informational content of the dance type from the perspective of the follower bee.
| dance type | informational content |
|---|---|
| OV-dance | dancers collect the familiar odour, and indicate the location of the known feeder (‘same-situation’). ‘Familiar odour’ means that followers know this odour from visits at the feeder during the training session. OV indicates that followers know both the odour (O) and the indicated location (vector, V). |
| NN-dance | dancers collect the unfamiliar odour and indicate the location of the unknown feeder (‘same-situation’). ‘Unfamiliar odour’ means that followers never collected solution containing this scent at our feeders. NN refers to ‘no field experience’ with either the odour or the location. |
| O-dance | dancers collect the familiar odour (O), but indicate the location of the unknown feeder (‘changed-situation’). |
| V-dance | dancers collect the unfamiliar odour, but indicate the known feeder (‘changed-situation’). V indicates that followers know the indicated location (V). |
(b) Behavioural observations
We quantified the time and the type of interaction between the employed foragers and the individually marked and treated inactive foragers inside the hive. The types of interaction were as follows:
Dance following. Bees that are located around the dancing bee within one bee length of the dancer, facing the dancer and moving so that her head stayed facing the dancer during dance circuits (Biesmeijer & Seeley 2005). The number of waggle runs followed was recorded.
Trophallaxis. Mouth-to-mouth contacts between active (incoming) foragers and treated inactive foragers. The active forager opens her mandibles and regurgitates a drop of solution between her mouthparts; the receiver protrudes her tongue towards the mandibles of the donor and tries to drink the solution.
(c) Statistical analyses
For data analysis, we used generalized linear mixed-effect models (GLMM) in R v. 2.5.1 (R Development Core Team 2006). R fitted the models using the lme4 package (Bates 2007). We used hive and trial as random effects and dance type or odour situation as fixed effects. All dependent variables had a Poisson distribution. In order to test for the significance of a fixed effect, we compared the model containing the fixed effect with the model without fixed effect. A likelihood ratio test then compared the two models (Faraway 2006) when the fixed effect had more than two levels; pair wise comparisons between levels were performed when a significant overall effect was found. We corrected for multiple testing of a dataset and adjusted the significance level by using the sequential Bonferroni method (Sokal & Rohlf 1995). Descriptive statistics are given as mean±s.e.
3. Results
(a) Private versus social information
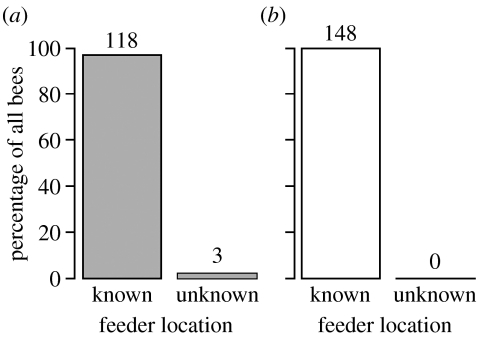
In both the same-situation and the changed-situation trials, most experienced foragers were reactivated to visit the feeder location they knew from previous field trips (figure 2). Only in H3, in the same-situation trial, three foragers arrived at the unknown feeder location. These three foragers followed dances of the opposite forager group (NN-dances) for 12, 19 and 22 waggle runs, respectively, and are the only bees for which we have clear evidence that the vector information provided by the waggle dance was used. Thirty bees followed the dancers that were collecting the known odour but indicated the unknown feeder location (O-dances). Of those, 26 bees landed at the feeder location where they previously experienced the food odour; none of the bees were captured at the feeder indicated by the vector information of the dance (table 2) and 18 bees followed the dancers carrying the unknown odour and indicating the known feeder (V-dance). Later, all of them were captured at the known feeder location. The same was true for bees following OV-dances. In summary, 43 bees followed dances indicating the unknown feeder location (17 NN-dances and 26 O-dances). Only three (7%) used this information.
Figure 2.
Arrivals of the reactivated foragers. The percentage of bees being captured at the known and unknown feeder locations in both (a) the same-situation (grey bars) and (b) changed-situation (white bar). Numbers above bars represent the bees.
Table 2.
Number of bees following the different types of waggle dances and the number of bees captured at the two feeders. (Overall difference between the different types of dances (OV, O, NN and V) with respect to the number of waggle runs followed per forager (Χ32=26.6, p<0.001).)
| dance type | n | bees captured | waggle runs followed | reactivation delay (min) | feeder location | |
|---|---|---|---|---|---|---|
| known | unknown | |||||
| OV | 41 | 36 | 4.68±2.7 | 3.73±3.3 | 36 | 0 |
| NN | 20 | 17 | 7.7±6.8 | 4.29±2.87 | 14 | 3 |
| O | 30 | 26 | 5.07±3.9 | 3.93±3.6 | 26 | 0 |
| V | 18 | 18 | 2.53±2.3 | 4.29±4.22 | 18 | 0 |
(b) Dance choice
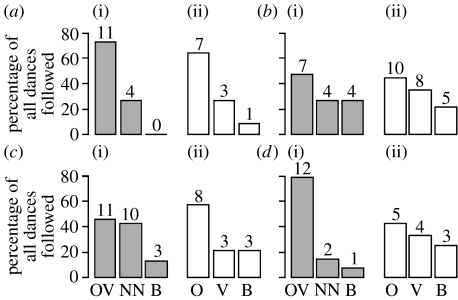
Figure 3 shows the number of experienced foragers following the different dance types in both situations. In all eight trials, more bees followed dances with familiar odours (54% of all cases) than dances with unfamiliar odours (30.2% of all cases); 15.8% of bees followed dances of both kinds (B-group). A GLMM showed that bees preferred to follow a dancer that collected food with a familiar food odour when compared with a dancer with an unfamiliar odour (GLMM, Χ12=10.2, p=0.001; figure 3). There was no difference between dances indicating the known feeder location and those indicating the unknown feeder location (Χ12=0.74, p=0.39), that is, we found no indication of an effect of the vector information on the dance choice.
Figure 3.
Behaviour of the dance followers. The percentage of bees following dances of a particular type in both situations (grey for (i) ‘same-situation’ and white for (ii) ‘changed-situation’). OV, NN, O and V are defined in table 1; B, experienced foragers follow both types of dances of a given situation. Numbers above bars represent the bees. (a) Hive 1, (b) hive 2, (c) hive 3 and (d) hive 4.
(c) Waggle runs followed
Reactivated bees, which have been filmed following dances, followed 4.6±3.38 waggle runs (n=106, range: 1–17). A to total of 62 bees (58.5%) followed fewer than five waggle runs and 44 bees (41.5%) followed at least five waggle runs. From this last category, 11 bees (10.4%) followed at least 10 waggle runs and 2 bees (1.9%) followed more than 15 waggle runs.
The attractiveness of a dance type may be apparent also on a second level, the total number of waggle runs followed per experienced forager for the different dance types (table 2). First, we tested for an overall effect of dance type (OV, O, NN and V) and found a significant effect (Χ32=26.6, p<0.001). Table 3 shows the comparisons between the different dance types. When looking at dances promoting the known odour, there was no difference in the number of waggle runs followed by experienced foragers between those that indicated the known location (OV-dance) and those indicated the unknown location (O-dance). On the other side, dancers carrying both unknown vector and unknown odour (NN-dance) were followed for more waggle runs than those carrying the known odour and indicating the known vector (OV-dance). In the reverse situation, dancers carrying the familiar odour (O-dance) were followed longer than those indicating the known vector (V-dance).
Table 3.
Comparisons between the different types of dances with respect to the number of waggle runs followed per bee; d.f.=1. (*Lower numbers compared with table 2 because in a few cases it was not possible to record the exact numbers of waggle runs followed. **Significant after sequential Bonferroni correction. Italics indicate p<0.025.)
| comparison | total n | Χ2-value | p-value |
|---|---|---|---|
| OV versus NN | 38*/20 | 9.47 | 0.002** |
| OV versus O | 38*/30 | 0.63 | 0.43 |
| OV versus V | 38*/17* | 3.47 | 0.062 |
| NN versus O | 20/30 | 0.04 | 0.84 |
| NN versus V | 20/17* | 1.97 | 0.16 |
| O versus V | 30/17* | 15.5 | <0.001** |
(d) Reactivation delay
It might be argued that more bees used the vector information of the dance and tried unsuccessfully to find the indicated feeder location. After failing to find the correct feeding site, these foragers would have then flown to the known feeder location. If this were true, one would expect that the time delay between dance following and capture at the feeder would be longer in the case of dancers providing conflicting information compared with those providing no conflict (OV- versus O-dance). However, we found no significant differences between foragers following different dance types (Χ32=1.67, p=0.64).
(e) Trophallactic interactions among foragers
We tested whether field experiences with a particular food odour affect the occurrence of trophallaxis between the inactive and active foragers. Overall, we recorded 74 trophallactic begging contacts of inactive foragers with active foragers returning with a familiar scent and 39 trophallactic begging contacts with the foragers returning with the unfamiliar food scent. Thus, inactive foragers were more likely to receive food from a forager offering an odour, which the inactive forager had previously learnt in the field (Χ12=11.0, p<0.001).
(f) Reactivation success
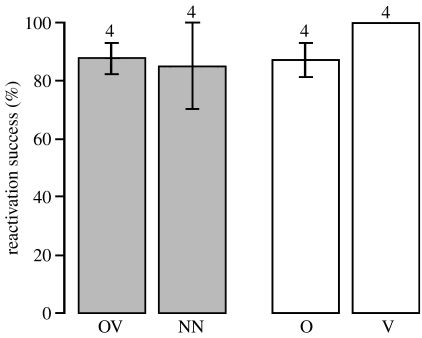
von Frisch (1946, 1967) stated that the location information provided by a dancing bee is important for the reactivation success of the dancer. Therefore, we measured the reactivation success of different dance types, that is, the proportion of foragers flying back to the known feeder after interacting with a dancing bee in the hive. Irrespective of whether followers knew the odour carried or the vector indicated by a dancer, reactivation success was above 80% for all types of dances in both situations (figure 4) and no significant difference was found between the dance types with respect to the reactivation success (Χ32=4.69, p=0.20).
Figure 4.
Reactivation success for the different dance types. Bars represent the percentage of bees (mean±s.e.) that were captured at the familiar feeder after following a dance of a certain type in the ‘same-situation’ (grey) and the ‘changed-situation’ (white). Numbers above the bars represent the four hives.
4. Discussion
In 93% of all cases when bees were following dances providing spatial information that diverged from their private navigational information, followers subsequently relied on self-acquired information. These results suggest that foragers with self-acquired (private) information about the location of profitable food sources are usually not interested in the vector information transmitted by the dancers, even if they follow dances thoroughly. Of all reactivated bees, 41.5% followed at least five waggle runs and 10.4% followed at least 10 waggle runs. One bee was captured at the known feeder after following 17 waggle runs of a dancer that indicated the unknown location. This is remarkable given that followers can decode the vector information after following only five waggle runs (von Frisch & Jander 1957). Hence, dances are often attended longer than would be necessary to identify the scent carried by the dancer or to decode the location information. The dance probably arouses the foraging motivation of the followers and some bees might need more stimulation until they leave the hive.
None of the 26 foragers followed the instructions of the dancers when dancers collected a scent, which the foragers had learnt at a different location (O-dance). Surprisingly, the food scent does not seem to be essential either. Of all bees, 82% following dancers indicating an unknown location and collecting an unfamiliar scent (NN-dance) were also reactivated and flew to the previously visited feeding site. The simple presence of a dancing bee motivates some foragers to fly to previously visited food patches, irrespective of vector and food odour information. Behaviourally active chemicals produced and released onto the cuticular surface of the dancer and into the air might alert experienced inactive foragers to generally good foraging conditions (Thom et al. 2007). However, since bees are attracted to dancers, which collected food odours they had previously learnt in the field, food odours seem to speed up the reactivation process. Such a preference to follow dancers that carry a known odour has already been shown for round dances (von Frisch 1923). The experience of an odour/reward combination causes an attraction to the odour in different behavioural contexts (e.g. von Frisch 1967; Koltermann 1969; Goyret & Farina 2005; Arenas et al. 2007).
It seems puzzling that dance followers ignore the spatial information of the dance because the dancer obviously advertises a high-quality food source (von Frisch 1967; Seeley 1995) while there is no guarantee that reactivated foragers will find food of similar quality at previously visited sites. On the other hand, many flower species offer nectar at particular periods during the day (von Frisch 1967; Vogel 1983) and there is a high probability that flowers of a given species offer nectar synchronously at different places. Furthermore, if the chance of finding previously visited food patches is considerably higher than the chance of finding the advertised flower patch, then it could still be worthwhile to fly to a potentially inferior but known food patch.
Neither the average numbers of waggle runs followed by foragers (table 2) nor reactivation success of dances differed for dancers that collected familiar scents but indicated unknown food locations (O-dances: 87.2% reactivation success) and dancers providing attuned information (OV-dances: 87.9% reactivation success). Thus, we cannot confirm von Frisch's (1967, 1946) statement that dances providing mismatched vector information are of little interest to experienced foragers and have a much lower reactivation success (37.5% compared with 92% in his experiment). In von Frisch's experiments, foragers either collected unscented food or he used two forager groups, which performed either round or waggle dances. Foragers might perceive round and waggle dances as intrinsically different. Our waggle data are to some degree ambiguous, because dances indicating the known feeder location (OV- versus NN-dance and V- versus O-dance) were followed for less time than the dances indicating the unknown feeder location. It is doubtful that this result is ecologically relevant since foragers flew back to the previously visited feeder in most cases.
We also found that the occurrence of trophallactic contacts was affected by olfactory field experiences. This could be a consequence of bees preferring to receive food containing a known food scent (Goyret & Farina 2005). Since dancers frequently distribute food samples to their followers (von Frisch 1967), it could also be a by-product of the preference to follow dancers that collected food with a known scent.
The transfer of vector information from dancers to followers has clearly been demonstrated (von Frisch 1967; Riley et al. 2005). It is normally assumed that foragers use the dance language when finding a food source after following a dance (e.g. Seeley 1983; Seeley & Visscher 1988). Biesmeijer & Seeley (2005) assumed that the location information provided by dancers is used whenever foragers follow at least 5–10 waggle runs. However, in our experiment, a substantial number of bees fell into this range, and most bees relied on self-acquired navigational information. So when do foragers actually use the dance language? The three cases of bees that apparently used the vector information strongly suggest that bees can switch their strategy. These bees followed on average 17 waggle runs that are similar to the number of runs reported by Michelsen (2003). We would expect that foragers switch their strategy and start preferring the social information if either the quality of the private information or the quality of the visited food patch is below a certain threshold. Information could be of low quality if it is outdated or not reliable (Leadbeater & Chittka 2007); a food patch is of low quality if it is of low relative profitability (Seeley 1995). Accordingly, van Bergen et al. (2004) showed that the quality of private information influences the use of social information in a situation of conflicting information in nine-spined sticklebacks (Pungitius pungitius). Hence, we would expect that the location information of the waggle dance is more relevant for the recruitment of foragers without a robust memory of foraging sites, because either they are new to foraging or their foraging activity was interrupted for longer periods.
Experiments investigating the waggle dance are often performed at the end of the flowering season (e.g. Riley et al. 2005; this study) or at places where there are few alternative food sources, because it is otherwise difficult to train bees to artificial feeders (Seeley 1995). In such environments, bees might use the vector information of the waggle dance more often than during times of nectar abundance in spring and summer, because private information is likely to be outdated and natural food patches are of lower quality. This would help to explain why colonies with misdirected dances often perform equally well in temperate habitats during times of nectar abundance (Sherman & Visscher 2002; Dornhaus & Chittka 2004).
However, even if follower bees often ignore the vector information of the waggle dance, the long-term consequences of the vector information are not well understood. If bees are recruited to a food patch and subsequently forage for several days (up to 21 days at the same patch; Ribbands 1949), then even these rare events might be of considerable ecological importance. The question of when honeybees use either private or social information under natural conditions needs further examination. This will most certainly reveal that the waggle dance modulates collective foraging in more complex ways than is currently assumed.
Acknowledgments
We thank Gonzalo Corti Bielsa, Gabriela Ramírez, Severine Loosli, Dolores Schütz and Francisca Segers for their help with data collection; Daniel Rankin, Francisca Segers and two anonymous referees for their comments on earlier versions of the manuscript; Michael Taborsky for logistic support; and Hector Verna and Peter Stettler for their technical help. C.G. was financed by the Janggen-Pöhn Stiftung and the Berner Hochschulstiftung. This study was supported by funds from ANPCYT, CONICET and University of Buenos Aires.
References
- Arenas A, Fernández V.M, Farina W.M. Floral odor learning within the hive affects honeybees' foraging decisions. Naturwissenschaften. 2007;94:218–222. doi: 10.1007/s00114-006-0176-0. doi:10.1007/s00114-006-0176-0 [DOI] [PubMed] [Google Scholar]
- Bates, D. 2007 lme4: linear mixed-effects models using S4 classes R package version 0.99875–7.
- Biesmeijer J.C, Seeley T.D. The use of waggle dance information by honey bees throughout their foraging careers. Behav. Ecol. Sociobiol. 2005;59:133–142. doi:10.1007/s00265-005-0019-6 [Google Scholar]
- Diaz P.C, Grüter C, Farina W.M. Floral scents affect the distribution of hive bees around dancers. Behav. Ecol. Sociobiol. 2007;61:1589–1597. doi:10.1007/s00265-007-0391-5 [Google Scholar]
- Dornhaus A, Chittka L. Evolutionary origins of bee dances. Nature. 1999;401:38. doi:10.1038/43372 [Google Scholar]
- Dornhaus A, Chittka L. Why do honey bees dance? Behav. Ecol. Sociobiol. 2004;55:395–401. doi:10.1007/s00265-003-0726-9 [Google Scholar]
- Faraway J.J. Chapman & Hall/CRC; Boca Raton, FL: 2006. Extending the linear model with R. [Google Scholar]
- Farina W.M, Grüter C, Diaz P.C. Social learning of floral odours within the honeybee hive. Proc. R. Soc. B. 2005;272:1923–1928. doi: 10.1098/rspb.2005.3172. doi:10.1098/rspb.2005.3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyret J, Farina W.M. Non-random nectar unloading interactions between foragers and their receivers in the honeybee hive. Naturwissenschaften. 2005;92:440–443. doi: 10.1007/s00114-005-0016-7. doi:10.1007/s00114-005-0016-7 [DOI] [PubMed] [Google Scholar]
- Grüter C, Acosta L.E, Farina W.M. Propagation of olfactory information within the honeybee hive. Behav. Ecol. Sociobiol. 2006;60:707–715. doi:10.1007/s00265-006-0214-0 [Google Scholar]
- Hrncir M, Mateus S, Nascimento F.S. Exploitation of carbohydrate food sources in Polybia occidentalis: social cues influence foraging decisions in swarm-founding wasps. Behav. Ecol. Sociobiol. 2007;61:975–983. doi:10.1007/s00265-006-0326-6 [Google Scholar]
- Johnson D.L. Communication among honey bees with field experience. Anim. Behav. 1967;15:487–492. doi: 10.1016/0003-3472(67)90048-6. doi:10.1016/0003-3472(67)90048-6 [DOI] [PubMed] [Google Scholar]
- Koltermann R. Lern- und Vergessensprozesse bei der Honigbiene—aufgezeigt anhand von Duftdressuren. Zeitschrift für vergleichende Physiologie. 1969;63:310–334. doi:10.1007/BF00298165 [Google Scholar]
- Leadbeater E, Chittka L. Social learning in insects—from miniature brains to consensus building. Curr. Biol. 2007;17:R703–R713. doi: 10.1016/j.cub.2007.06.012. doi:10.1016/j.cub.2007.06.012 [DOI] [PubMed] [Google Scholar]
- Lindauer M, Kerr W.E. Communication between the workers of stingless bees. Bee World. 1960;41:29–71. [Google Scholar]
- Michelsen A. Signals and flexiblity in the dance communication of honeybees. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2003;189:165–174. doi: 10.1007/s00359-003-0398-y. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2006. R: a language and environment for statistical computing. [Google Scholar]
- Reinhard J, Srinivasan M.V, Guez D, Zhang S.W. Floral scents induce recall of navigational and visual memories in honeybees. J. Exp. Biol. 2004;207:4371–4381. doi: 10.1242/jeb.01306. doi:10.1242/jeb.01306 [DOI] [PubMed] [Google Scholar]
- Ribbands C.R. The foraging method of individual honey-bees. J. Anim. Ecol. 1949;18:47–66. doi:10.2307/1581 [Google Scholar]
- Ribbands C.R. Communication between honeybees. I: the response of crop-attached bees to the scent of their crop. Proc. R. Entomol. Soc. Lond. A. 1954;29:141–144. [Google Scholar]
- Riley J.R, Greggers U, Smith A.D, Reynolds D.R, Menzel R. The flight paths of honeybees recruited by the waggle dance. Nature. 2005;435:205–207. doi: 10.1038/nature03526. doi:10.1038/nature03526 [DOI] [PubMed] [Google Scholar]
- Seeley T.D. Division of labor between scouts and recruits in honeybee foraging. Behav. Ecol. Sociobiol. 1983;12:253–259. doi:10.1007/BF00290778 [Google Scholar]
- Seeley T.D. Harward University Press; Cambridge, MA: 1995. The wisdom of the hive: the social physiology of honey bee colonies. [Google Scholar]
- Seeley T.D, Visscher P.K. Assessing the benefits of cooperation in honeybee foraging: search costs, forage quality, and competitive ability. Behav. Ecol. Sociobiol. 1988;22:229–237. doi:10.1007/BF00299837 [Google Scholar]
- Sherman G, Visscher P.K. Honeybee colonies achieve fitness through dancing. Nature. 2002;419:920–922. doi: 10.1038/nature01127. doi:10.1038/nature01127 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. 3rd edn. W. H. Freeman and company; New York, NY: 1995. Biometry. [Google Scholar]
- Thom C, Gilley D.C, Hooper J, Esch H.E. The scent of the waggle dance. PLoS Biol. 2007;5:e228. doi: 10.1371/journal.pbio.0050228. doi:10.1371/journal.pbio.0050228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen Y, Coolen I, Laland K.N. Nine-spined sticklebacks exploit the most reliable source when public and private information conflict. Proc. R. Soc. B. 2004;271:957–962. doi: 10.1098/rspb.2004.2684. doi:10.1098/rspb.2004.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. Ecophysiology of zoophilic pollination. In: Lange O.L, Nobel P.S, Osmond C.B, Ziegier H, editors. Physiological plant ecology III. Springer; Berlin, Germany; New York, NY: 1983. pp. 559–624. [Google Scholar]
- von Frisch K. Über die “Sprache” der Bienen. Zoologisches Jahrbuch (Zoologie und Physiologie) 1923;40:1–186. [Google Scholar]
- von Frisch K. Die Tänze der Bienen. Österreichische Zoologische Zeitschrift. 1946;1:1–48. [Google Scholar]
- von Frisch K. Harvard University Press; Cambridge, MA: 1967. The dance language and orientation of bees. [Google Scholar]
- von Frisch K, Jander R. Über den Schwänzeltanz der Bienen. Zeitschrift für vergleichende Physiologie. 1957;40:239–263. doi:10.1007/BF00340570 [Google Scholar]
- von Frisch K, Rösch G.A. Neue Versuche über die Bedeutung von Duftorgan und Pollenduft für die Verständigung im Bienenvolk. Zeitschrift für vergleichende Physiologie. 1925;4:1–21. doi:10.1007/BF00341784 [Google Scholar]
- Wells P.H, Wenner A.M. Do honey bees have a language? Nature. 1973;241:171–175. doi:10.1038/241171a0 [Google Scholar]
- Wenner A.M, Johnson D.L. Simple conditioning in honey bees. Anim. Behav. 1966;14:149–155. doi: 10.1016/s0003-3472(66)80023-4. doi:10.1016/S0003-3472(66)80023-4 [DOI] [PubMed] [Google Scholar]
- Wenner A.M, Wells P.H. Columbia University Press; New York, NY: 1990. Anatomy of a controversy: the question of a “language” among bees. [Google Scholar]