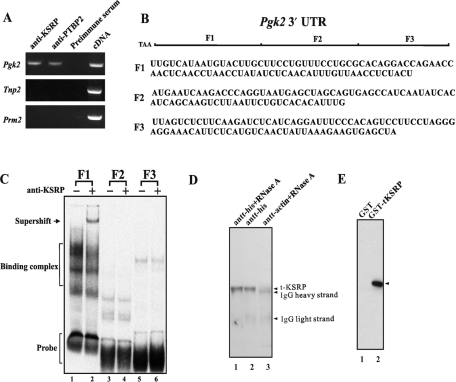
Figure 2.
KSRP binds to Pgk2 mRNA in the testis and in vitro to the F1 region of the Pgk2 mRNA and to PTBP2. (A) Messenger RNA detection by RT–PCR of purified germ cell mRNAs immunoprecipitated from mouse testis extracts with anti-KSRP. (B) A diagram of the three subclones (F1, F2 and F3) of the Pgk2 3′-UTR. (C) KSRP selectively binds to the F1 region to the 3′-UTR of Pgk2 mRNA. Testis extracts (20 µg) were incubated with [32P]-labeled transcripts generated by in vitro transcription from subclones (F1, F2 and F3) of the 3′-UTR of Pgk2 mRNA as previously described (9). RNA–protein complexes were resolved in 4% polyacrylamide gels in 0.5× TBE buffer. Anti-KSRP was added to indicated extracts before the addition of the radiolabeled RNAs. (D) Western blot of endogenous t-KSRP coimmunoprecipitated with r-PTBP2 from testis extracts. His-tagged recombinant PTBP2 (20 µg) was incubated with His-tag affinity resins and testis extract (1 mg). Lane 1, plus RNase A; lane 2, no RNAse; lane 3, antiactin control. Following centrifugation, pellets were solubilized and the proteins resolved in 10% SDS–PAGE. KSRP was detected by western blotting. (E) Recombinant t-KSRP and recombinant PTBP2 interact. GST-tagged r-KSRP was incubated with r-PTBP2. Following the addition of glutathione agarose beads and centrifugation, the pellets were solubilized, electrophoresed in 10% SDS–PAGE and PTBP2 was detected by western blotting. Lane 1, GST; lane 2, t-KSRP tagged with GST.