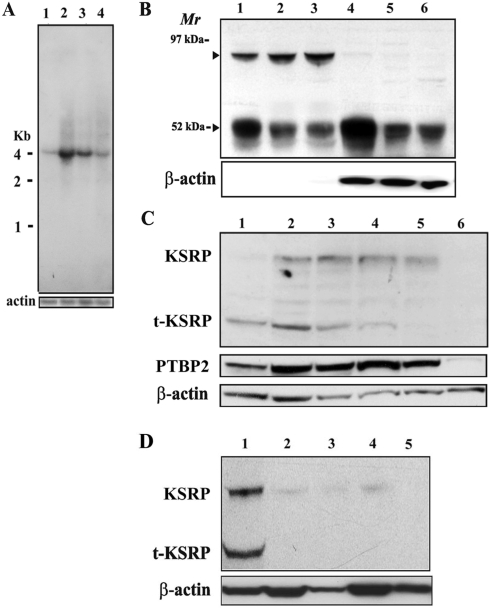
Figure 4.
Two isoforms of KSRP, KSRP and t-KSRP, are expressed from one mRNA in the testis, and t-KSRP is enriched in the cytoplasm of early-stage meiotic cells. (A) Northern blot of KSRP mRNA from brain and prepuberal and adult testes. Total RNAs (15 µg) were electrophoresed in 1% agarose–formaldehyde gels, transferred to Hybond-N + membranes and hybridized with a KSRP 3′-UTR probe. Rehybridization of the blot with a coding region probe for actin served as a loading control. Lane 1, adult brain; lane 2, 17-day-old testis; lane 3, 22-day-old testes; lane 4, adult testes. (B) Western blot of KSRP in nuclear and cytoplasmic fractions of prepuberal and adult testes. Proteins (20 µg) extracted from purified nuclei and cytoplasm were separated by 10% SDS–PAGE and blotted onto polyvinylidene difluoride membranes. Anti-KSRP was used at a dilution of 1:3000. Antiactin served as a loading control. Lanes 1–3 and 4–6 were extracts from nuclei and cytoplasm, respectively. Lanes 1 and 4, 17-day-old testis; lanes 2 and 5, 22-day-old testes; lanes 3 and 6, adult testes. (C) Western blot of KSRP, PTBP2 and actin in extracts of isolated populations of germ cells. Lane 1, preleptotene spermatocytes; lane 2, leptotene/zygotene spermatocytes; lane 3, early pachytene spermatocytes; lane 4, pachytene spermatocytes; lane 5, round spermatids; lane 6, condensing spermatids/residual bodies. The preleptotene spermatocytes, leptotene/zygotene spermatocytes and early pachytene spermatocytes were isolated from the testes of 17-day-old mice, whereas the pachytene spermatocytes, round spermatids and condensing spermatids/residual bodies were isolated from the testes of adult mice. (D) Western blot of t-KSRP in tissue extracts. Lane 1, testis; lane 2, brain; lane 3, liver; lane 4, kidney; lane 5, lung protein extracts (30 µg) analyzed as in (B) with actin serving as a loading control.